This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Using operando Raman spectroscopy to investigate converting carbon dioxide to ethanol on Ag nanowires
In a study, published in the journal Science China Chemistry and led by Prof. Pingping Fang (School of Chemistry, Zhejiang University) and Prof. Jianfeng Li (College of Chemistry and Chemical Engineering, Xiamen University), experiments were performed by using an Xplora Raman spectrometer with a 50x microscope objective and an excitation wavelength of 638 nm from a He–Ne laser.
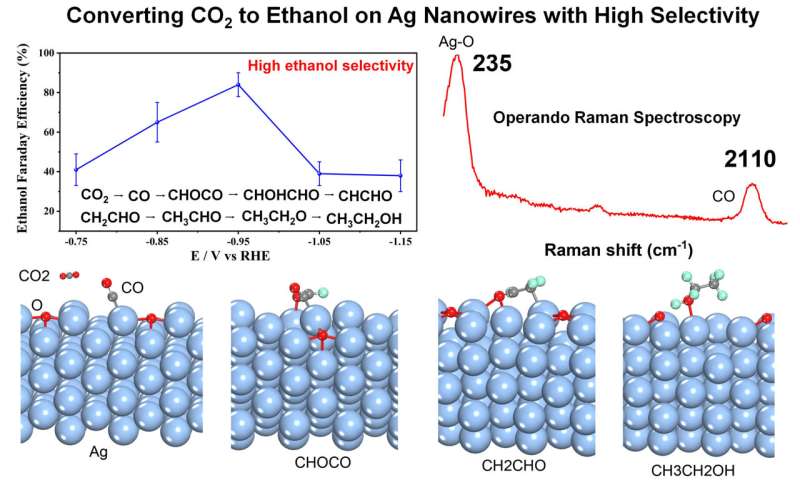
"Due to the proper adsorption energy of CO on the partially oxidized Ag NWs, it is of great significance to correlate the high ethanol selectivity for CO2 electroreduction with the partially oxidized active center. Ag catalysts have been shown to exhibit high selectivity for CO2 electroreduction to CO at low over potentials with depressed H2 evolution. CO is an important intermediate for C-C coupling, therefore, Ag has the potential to exhibit high selectivity for ethanol products by tuning the adsorption energy of CO. This study provides a new insight to design efficient catalysts and investigate the mechanisms to improve the selectivity," Fang says.
Interestingly, high ethanol FE was obtained on partially oxidized Ag NWs for CO2 electroreduction, and operando EC-SERS combined with DFT calculation explained the mechanisms why partially oxidized Ag NWs exhibited so high ethanol selectivity. The ethanol FE can reach as high as 85% on partially oxidized Ag NWs at −0.95 V vs. RHE. Operando EC-SERS found the high coverage of CO can greatly facilitate the ethanol formation on partially oxidized Ag NWs during CO2 electroreduction.
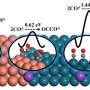
DFT calculation results show that the adsorption energy of CO on the partially oxidized Ag NWs is higher than that on Cu, and the reaction free energy of CO coupling with *CHO to *COCHO intermediate on partially oxidized Ag NWs is smaller than that on Cu surface, which explains the high ethanol selectivity very well.
Therefore, experiment results, operando EC-SERS and DFT calculations together prove that such partially oxidized Ag NWs can provide high ethanol selectivity for CO2 electroreduction. These results provide new clues for designing Ag based catalysts to improve the ethanol selectivity and mechanism studies.
More information: Qiong Liu et al, Converting CO2 to ethanol on Ag nanowires with high selectivity investigated by operando Raman spectroscopy, Science China Chemistry (2022). DOI: 10.1007/s11426-022-1460-7
Provided by Science China Press