This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Cu/CuNC dual-site interface promotes carbon dioxide electroreduction to ethanol
Reducing the energy consumption is one of requirements for scalable electrochemical energy conversion and electrosynthesis, including electrochemical reduction of CO2 (ECR) into chemicals.
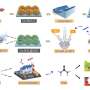
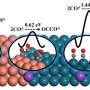
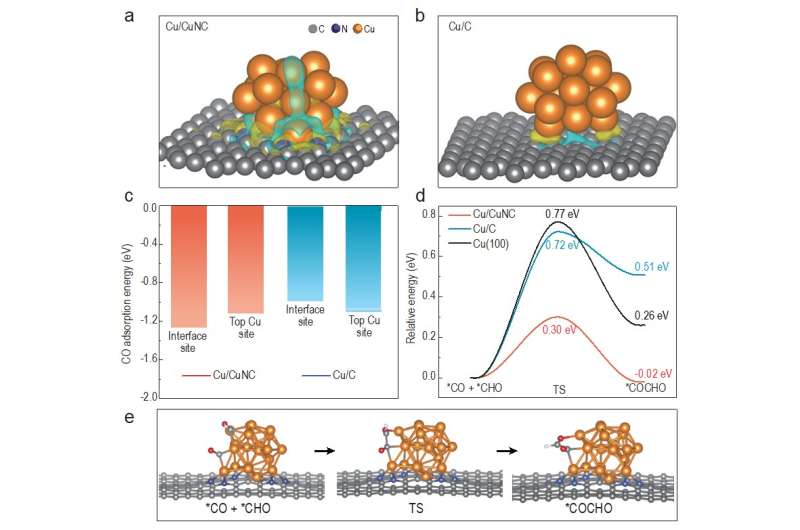
This study, published in the journal National Science Review and led by Prof. Jin-Song Hu (Institute of Chemistry, Chinese Academy of Sciences) and Prof. Jinlan Wang (School of Physics, Southeast University), aims at reducing the overpotential for ECR. The theoretical calculation results were firstly performed, suggesting that the asymmetric electronic structure on the Cu/CuNC interface could significantly enhance the adsorption of *CO intermediate and reduce the reaction energy barrier of the C-C coupling, thereby improving the selectivity of the ethanol at a low overpotential.
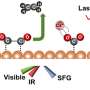
The researchers subsequently synthesized such a catalyst with a high density of Cu/CuNC interfacial sites by the in-situ electrochemical reduction of highly loaded CuNC SACs. Ex-situ spectroscopic characterization results evidenced that the nano-sized Cu nanoparticles (~3.6 nm) were formed and surrounded by nitrogen (N).
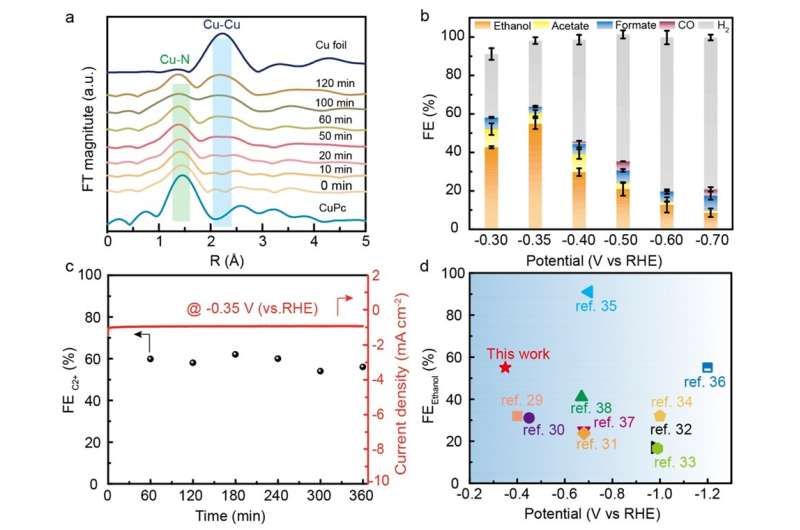
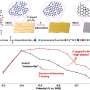
In-situ spectroscopic characterization results revealed the formation process of the Cu/CuNC interfacial sites and the coexistence of zero-valence Cu and Cu-N-C sites. The ECR tests showed that the ER-Cu/CuNC catalyst exhibited an excellent Faradaic efficiency of 60.3% for C2+ (55% for FEethanol) at a low potential of -0.35 V vs. RHE, which is better than most reported Cu-based catalysts.
In addition, the systematical control experiments indicated that the catalysts without Cu/CuNC interfacial sites delivered a negligible ethanol production rate, corroborating the critical role of the Cu/CuNC interfacial sites in promoting C-C coupling at low overpotential. These findings provided new insights and attractive approaches to creating multisite interface as highly efficient catalytic centers for promoting ECR to C2+ products at low energy cost.
More information: Yan Yang et al, In-situ Constructed Cu/CuNC Interfaces for Low-Overpotential Reduction of CO2 to Ethanol, National Science Review (2022). DOI: 10.1093/nsr/nwac248
Provided by Science China Press