This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Speeding up sugar's conversion into fuel

University of Queensland researchers have found a way to more efficiently convert sugarcane into a building block of aviation fuel and other products.
By zeroing in on a specific enzyme, a UQ team working in collaboration with the Technical University of Munich (TUM) has sped up the slowest step in processing sugar into a chemical called isobutanol.
Professor Gary Schenk from UQ's School of Chemistry and Molecular Biosciences said isobutanol from a renewable resource could be used to make fuels, plastics, rubbers and food additives.
"Our research into this particular enzyme means we can accelerate the production rate and yield of isobutanol from sugarcane, ultimately enabling biomanufacturers to make diverse products at scale sustainably and efficiently," Professor Schenk said.
"Usually during a biomanufacturing process, cells such as yeasts are used as a production platform, but in our research only a small number of a sugar acid-specific dehydratase enzyme was used.
"Having sugar-converting enzymes operate outside a cellular environment meant we could bypass many of the pitfalls of the more traditional cell-based biomanufacturing methods.
"This has led to much higher yields of isobutanol with fewer unwanted side products."
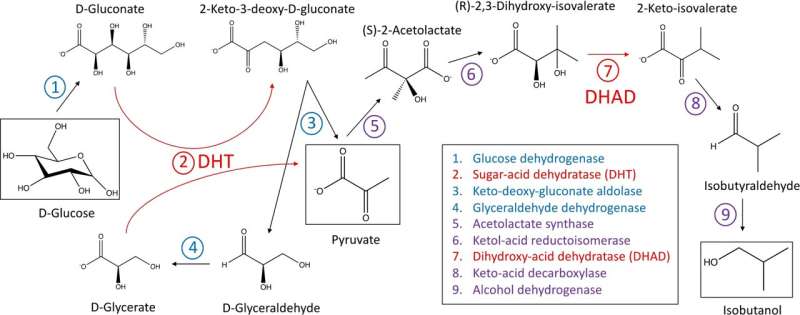
Cell-based production of isobutanol from sugar creates about 25 grams per liter of liquid cell culture but in the UQ study, the cell-free method produced at least 10 times that amount.
"The cell-free method gives biomanufacturers more control and results in much higher yields, meaning a higher return on their investment and a more sustainably produced product—it's a win-win," Professor Schenk said.
"One limitation in creating bulk products like aviation fuel with a cell-free process has been the availability of enough enzymes but developments in enzyme engineering and production mean large-scale production is now achievable.
"We're only at the dawn of what is a very exciting age in this space."
Professor Damian Hine from UQ's Queensland Alliance for Agriculture and Food Alliance said the research proved the enormous potential of cell-free biomanufacturing.
"While there have been commercial limitations to producing the enzymes, we now have enough evidence to show that large-scale manufacturing using the cell-free enzymes process is commercially viable and should play a major role in future biomanufacturing," he said.
This research is published in Chemistry—A European Journal.
More information: Tenuun Bayaraa et al, Structural and Functional Insight into the Mechanism of the Fe−S Cluster‐Dependent Dehydratase from Paralcaligenes ureilyticus, Chemistry—A European Journal (2022). DOI: 10.1002/chem.202203140
Journal information: Chemistry – A European Journal
Provided by University of Queensland