This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A robotic microsurgeon reveals how embryos grow
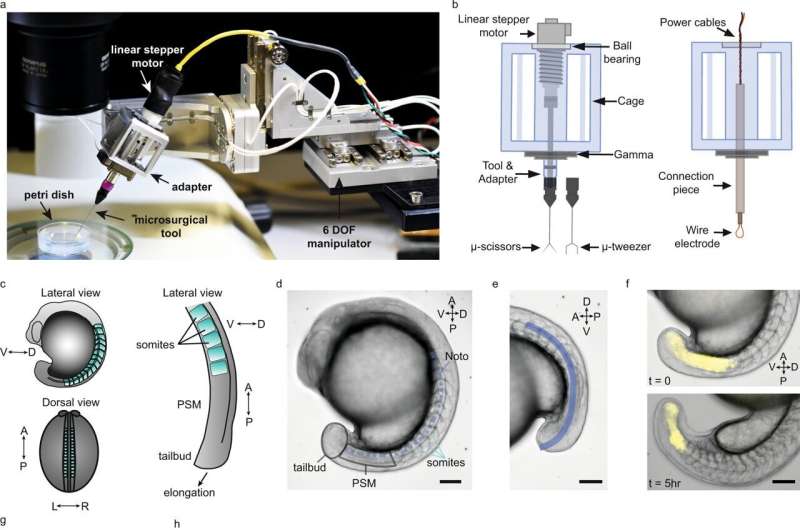
Combining biology and robotics, scientists at EPFL have built a robotic microsurgery platform that can perform high-precision, micrometer-resolution dissections to advance our understanding of how the vertebrate body forms during embryonic development.
Understanding the biology behind an embryo's development is crucial not only from a basic science perspective, but also from a medical one. However, we are in dire need for tools that can help us systematically and explore embryonic development.
"The original experimental approach in embryology is microsurgery," says Andy Oates at EPFL's School of Life Sciences. "But it used to be done with a very simple microscope and very simple tools like cactus spines or sharpened pieces of wire. Another problem is that we naturally have a tremor in our hands, which makes microsurgery difficult for some people. It takes years of training, and only some people can do it, so the throughput is very low."
Combining robotics and biology
In an effort to address the current limitations of microsurgery techniques, Oates joined forces with Professor Selman Sakar at the School of Engineering, an expert in microtechnology and small-scale robotics. "In my laboratory, we have been building robotic tools for tissue micro-manipulation," says Sakar.
"Together with Andy [Oates], we asked whether we could use some of these tools to facilitate research in embryology in general, to make it more reliable and give it a higher throughput, and in this case to specifically understand the biomechanics of how tissue morphogenesis [the shaping and structuring of a developing tissue] in zebrafish works."
The two professors received funding for an iPhD, a specialized doctoral fellowship at EPFL that combines life science research with another discipline. The iPhD candidate, Ece Özelçi, trained on both robotics and developmental biology.
"I think it's a great program, because, honestly, I would otherwise never have done such interdisciplinary research," she says. "It was quite intense; it's not like you only focus on a single discipline. I learned quite a lot from both fields, and I think it's a really great opportunity if you want to acquire a unique skill set."
A new robot-assisted platform
Publishing in Nature Communications, the researchers describe the new platform's role as "robot-assisted tissue micromanipulation." It is compact (200 x 100 x 70 mm3), high-resolution (4 nm position and 25 μ° rotation), and dexterous, with several degrees of freedom. The tool can position itself automatically without any manual intervention and do so with high, reproducible stability.
The researchers drew inspiration from related microsurgery systems from ophthalmology and neurology, which are also quite compact and precise, and also rely on microscopes, even though their target objects are often larger than an embryo.
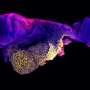
The scientists tested the platform's capabilities by using it to study body axis elongation of the zebrafish embryo. "Our lab focuses on how the backbone forms, and part of that is how the body elongates, grows out, and segments itself," says Oates.
"We use the zebrafish embryo as a model, and the idea is to look at the contribution of different parts of the embryo to the process of development. In this case, we look at how embryos elongate themselves and how they segment themselves, and how those two processes interact. Our approach is to physically separate elongation and segmentation by microsurgery, and see how each operates when the other process isn't there."
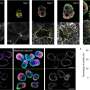
Using the platform, Özelçi and her colleagues were able to target precise regions of the zebrafish embryo. The robot-assisted microsurgery allowed them to remove the embryo's elongating tail and grow it separately—a process called explanting, which is often used in embryological research.
The study revealed a surprising behavior of the embryo's notochord, which acts as an early "backbone" for the larva when it begins to swim. "The notochord pushes so hard inside the tail that it can buckle itself," says Oates. "Normally the embryo would elongate uniaxially, but once we physically stopped the process, the notochord kept elongating, generating compressive stresses that led to buckling."
Biology for advancing engineering, and vice-versa
"In addition to embryology, our research enables us to reverse engineer the developmental programs for tissue engineering," says Sakar. "If we understand how forces lead to tissue morphogenesis, we could replicate these conditions with engineered tissues in vitro. Like biochemical factors, providing the right mechanical environment and signals is critical for the tissues to develop and function properly.
"We are also motivated to create biological machines that are designed to perform specific engineering tasks. For example, we would like to engineer mini-hearts that serve as organic pumps with much simpler architecture compared to the real heart. To this end, robot-assisted microsurgery provides not only the construction principles, but also provides the means to manufacture machines from the living matter through mechanically-guided self-assembly."
But will such platforms gain broader use? "I envision that such robotic micromanipulation tools will become instrumental in every life science laboratory," says Sakar. "Regardless of the chosen biological model system, ranging from single cells to organisms, robotics and automation can empower the scientists."
"Medical robots are quite advanced," he adds. "It is time to bring the unique capabilities of surgical robotics to the biomedical research community. Handling biological samples in an automated fashion will increase the throughput, precision, and repeatability of data acquisition while democratizing procedures that require fine skills and years of experience. Combined with intelligent imaging and microscopy, the possibilities are endless."
More information: Ece Özelçi et al, Deconstructing body axis morphogenesis in zebrafish embryos using robot-assisted tissue micromanipulation, Nature Communications (2022). DOI: 10.1038/s41467-022-35632-4
Journal information: Nature Communications
Provided by Ecole Polytechnique Federale de Lausanne