Protein disulfide isomerase regulates radio sensitivity by mitophagy
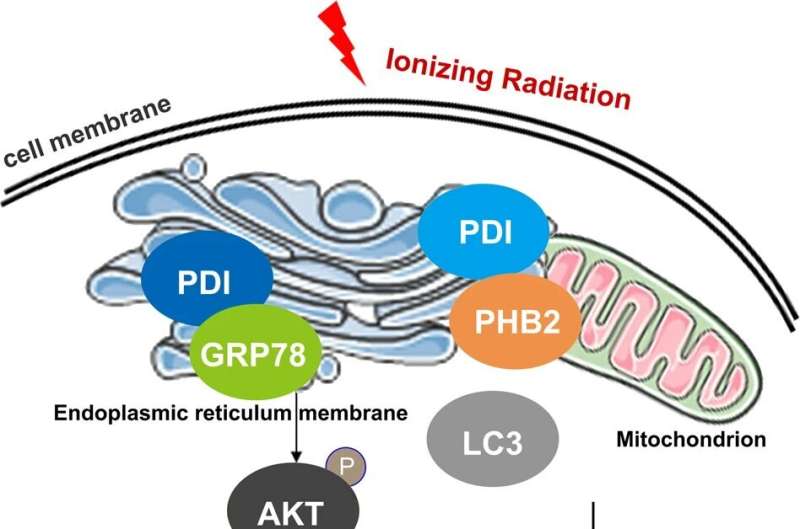
A research team led by Prof. Zhao Guoping from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences (CAS) has proposed a mechanism by which protein disulfide isomerase (PDI) can translocate from endoplasmic reticulum (ER) to the mitochondria under radiation stimulation, participate in regulating mitophagy and mediate tumor radiosensitivity, which is of great significance for understanding the role of mitochondrial targets in radiosensitivity.
This work was published in Cell Death & Disease.
Radiation therapy can induce mitochondrial damage and disrupt mitochondrial function, leading to a gradual accumulation of defective organelles. Mitophagy is a specific autophagy phenomenon that selectively eliminates damaged mitochondria. Moderate autophagy can promote cell homeostasis, whereas excessive autophagy can accelerate the induction of cell death.
PDI is an endoplasmic reticulum enzyme that mediates the formation of disulfide bonds. It is critical to the occurrence, development and treatment of cancer. The high expression of PDI in tumor tissue is usually accompanied by the increase of tumor pathological grade.
In this work, the researchers found that the risk of metastasis and poor prognosis of cancer patients would be increased by the high expression of PDI in colorectal cancer and other tumors significantly.
They found that the combination of PDI and GRP78 was enhanced mechanistically after ER stress. This combination, which inhibited the degradation of AKT by GRP78, eventually activated the mTOR pathway to inhibit autophagy initiation.
Aside from that, PDI can directly interact with the mitophagy receptor PHB2 in mitochondrial and then block the binding of LC3II and PHB2 competitively to inhibit the mitophagy signaling.
"These results indicate that PDI can reduce the radio sensitivity by regulating mitophagy, and clarify the new function of PDI in radiation-induced autophagy," said Wang Ruru, first author of the study.
This work provides new insights into the understanding of mitochondrial target to regulate tumor radiosensitivity, and provides a basic principle for the use of PDI inhibitors in clinical tumor radiotherapy.
More information: Ruru Wang et al, Protein disulfide isomerase blocks the interaction of LC3II-PHB2 and promotes mTOR signaling to regulate autophagy and radio/chemo-sensitivity, Cell Death & Disease (2022). DOI: 10.1038/s41419-022-05302-w
Provided by Chinese Academy of Sciences