Multiscale dynamical cross-talk in zeolite-catalyzed methanol-to-olefins reaction
Methanol-to-olefins (MTO) conversion, one of the most important reactions in C1 chemistry, has proven to be the most successful non-petrochemical industrialized routes for producing light olefins. Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has been dedicated to the R&D of the MTO reaction for the past four decades.
Based on DICP's MTO technology (DMTO), the world's first MTO unit was constructed and started up in 2010 in China. Since then, DICP has developed the second and the third-generation DMTO process (DMTO-II and DMTO-III) through continuous technological innovation. At present, MTO has become one of the important routes to produce ethylene and propylene in China.
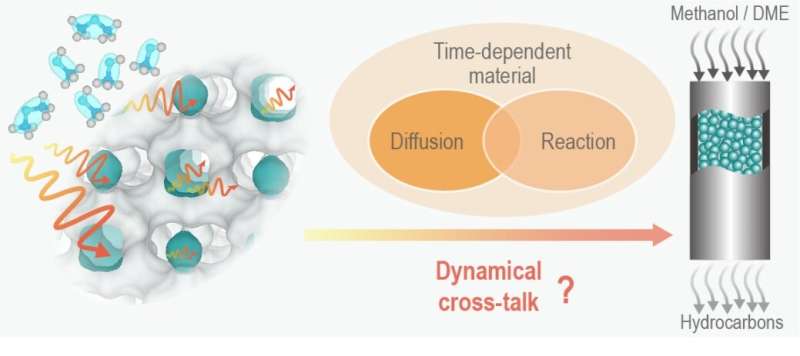
Establishing a comprehensive understanding of the dynamical multiscale diffusion and reaction process is crucial for zeolite shape-selective catalysis and is urgently demanded in academia and industry, especially for zeolite-catalyzed MTO conversion. So far, diffusion and reaction for MTO and DTO (dimethyl ether-to-olefins) have been usually studied separately and focused on a single scale.
The dynamic autocatalysis of MTO further complicates the reaction and diffusion in MTO reaction. It is challenging but imperative to unravel the dynamical interactive effect of diffusion and reaction within the time-dependent MTO zeolite materials at multiple scales.
In the recent study published in National Science Review, the research team led by Profs. Liu Zhongmin and Wei Yingxu (from National Engineering Laboratory for Methanol to Olefins, DICP, CAS) disclose the dynamical multiscale cross-talk behaviors and mechanisms among time-dependent material, diffusion and reaction for SAPO-34 catalyzed methanol and dimethyl ether conversions.
This work found that even being performed in the same zeolite material and possessing very close hydrocarbon pool mechanisms, the dynamical proceeding of the DTO reaction (relative to MTO) is distinctly regulated by the cross-talk of diffusion—reaction—material.
On one side, due to the confined-organics modification, cavity type SAPO-34 material is dynamically evolved in time with the autocatalytic MTO reaction running from the initiation to decay. On the other side, the time-dependent material, in turn, creates the catalysis with the dynamical evolution of diffusion and reaction.
Such dynamical cross-talk among time-dependent material, diffusion, and reaction occurs from the catalyst-bed scale to the catalyst crystal and CHA-cavity scale, and eventually results in the spatiotemporal heterogeneity in carbonaceous species distribution at multiple scales, revealing the root of heterogeneous catalytic efficiency, shape-selective catalysis (especially reactant shape-selective catalysis) and deactivation mode.
The multiscale cross-talk behaviors and mechanisms originate from the reactant shape-selectivity of zeolite materials in the dynamical reaction procedure of MTO and DTO. Compared with methanol, mass transfer of DME is constrained over SAPO-34, since its external surface permeation and inter-cavity hopping are hindered due to the higher energy barrier of surface permeability and intracrystalline diffusivity.
The constrained mass transfer of DME elongates the reaction zone of DTO over the catalyst bed, but also engenders the lower local chemical potential of the reactant, thereby generating moderate reaction kinetic and less heavier coke in the local catalyst microenvironment. Such cross-talk of diffusion—reaction—material occurring at microscale in the CHA cavity triggers the cross-talk behaviors at multiple scales: (i) enabling the inner part of the catalyst crystal still accessible to quite a part of DME at the late reaction stage, sustaining the turnover of DME with high capacity, and (ii) eventually leading to a relatively moderate and homogenous reaction and deactivation mode, and higher catalyst utilization efficiency.
In contrast, methanol conversion exhibits a layer-by-layer inhomogeneous reaction and deactivation mode and meanwhile, the higher local chemical potential of methanol enables the intensified reaction and deactivation localized in the outer part of the catalyst crystal, working as the main efficient zone. The characteristics of the DTO reaction presented in this work, moderately evolved reaction kinetics and depressed coke deposition, imply the possibility of realizing the long-term operation of fixed-bed DTO process.
Zeolite catalysis not only reflects the reaction characteristics of heterogeneous catalysis, but also provides enhanced, moderate or suppressed local reaction kinetics through the special catalytic microenvironment, which lead to the heterogeneity of diffusion and reaction at multiple scales, thereby realizing efficient and shape-selective catalysis.
For the specific dynamic reaction catalyzed by zeolite material, achieving the best spatiotemporal cooperation of material, diffusion and reaction is the most critical strategy for the optimized catalyst development and process application.
More information: Shanfan Lin et al, Multiscale dynamical cross-talk in zeolite-catalyzed methanol and dimethyl ether conversions, National Science Review (2022). DOI: 10.1093/nsr/nwac151
Provided by Science China Press