Scientist discovers new oxidation state of rhodium
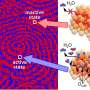
![Frontier natural molecular orbital plot at 0.1 e Bohr−3 (state-specific CASSCF(15,20)/aug-cc-pVTZ-DK) for the 1A1' electronic ground state in D3h point group symmetry of [RhO3]+. Credit: Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202207688 Scientist discovers new oxidation state of rhodium](https://scx1.b-cdn.net/csz/news/800a/2022/scientist-discovers-ne.jpg)
Mayara da Silva Santos, doctoral candidate at the University of Freiburg's Institute of Physics, has discovered a new oxidation state of rhodium. This chemical element is one of the most catalytically important platinum-group metals and is used, for example, in catalytic converters for automobiles.
Rhodium is actually already well studied. What helped Silva Santos make the rare discovery of a surprisingly high oxidation state was a new approach: As part of her doctoral dissertation, she is studying unusual transition metal oxides. Her discovery of so-called rhodium(VII) has just been published in Angewandte Chemie International Edition.
Oxides in an ion trap
"Discoveries are always exciting," says the Freiburg chemist. "Our oxides are highly reactive but could play an important role as reactive intermediate states."
They can be observed in chemical reactions only with difficulty because they are very short-lived. "We were able to store the oxides in a special ion trap at low temperatures over an extended time period and thus study them undisturbed," says Silva Santos.
Third-highest oxidation state of all elements
This interdisciplinary approach, combining physics and chemistry as well as experiment and theory, was the key to success, says Prof. Dr. Tobias Lau, professor at the University of Freiburg's Institute of Physics. "The teamwork between different disciplines was very helpful in sample production, mass spectrometry, X-ray spectroscopy, and data analysis."
An important finding of this interdisciplinary work is that more valance electrons than previously thought can take part in chemical bonds with rhodium and that rhodium can assume the third-highest oxidation state of all elements. This highest oxidation state of rhodium—rhodium(VII)—was previously unknown but could play a role in chemical reactions.
More information: Mayara da Silva Santos et al, The Highest Oxidation State of Rhodium: Rhodium(VII) in [RhO 3 ] +, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202207688
Journal information: Angewandte Chemie International Edition
Provided by University of Freiburg