First mouse model with mitochondrial tRNALeu mutation developed

Studying the role of mitochondria—the specialized structures within cells responsible for energy production—in metabolic diseases has been difficult because of a lack of animal models with the necessary mitochondrial mutations to observe these tiny organelles. However, a team from the University of Tsukuba have now generated the first mouse model carrying a disease-associated mitochondrial mutation and have shown that the resulting disease is caused by faulty RNA processing. Their study is published in Nucleic Acids Research.
Mitochondria are surrounded by a membrane and contain a small amount of their own DNA. This mitochondrial DNA codes for some components of the energy-generating machinery, as well as genes for both ribosomal RNAs (components of the machinery that makes proteins) and transfer RNAs that play a key role in protein synthesis. Mutations in the mitochondrial genome are known to be linked to some human disorders such as diabetes, neurodegenerative diseases, infertility, and cancer.
Researchers at the University of Tsukuba fused cells that contained mitochondria carrying mutant DNA, but no nucleus, with embryonic stem cells that had had all their mitochondria removed by a drug called rhodamine 6G, thus creating a mouse model containing the A2748G mutation. This mutation is found in human patients, where it is known as the A3302G mutation, and is one of the common mitochondrial mutations associated with some human diseases, such as certain neuromuscular diseases, encephalopathy (brain damage), and metabolic disorders.
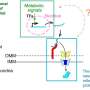
The mice carrying this mutant mitochondrial DNA developed metabolic disorders that mimicked the symptoms shown by human patients carrying the equivalent human mutation. This enabled further study to uncover the underlying molecular mechanism of the associated disease, which showed that this mutation affected the processing of RNAs by interfering with protein synthesis in the affected mice.
"The faulty processing of the RNA containing the A2748G mutation led to a decrease in the translation of a protein known as ND1," explains main author Professor Kazuto Nakada. "ND1 is a component of a protein complex known as Complex 1, the first of five key protein complexes in the process of energy generation known as oxidative phosphorylation." The resulting Complex I deficiency affected the function of the cellular energy-generating pathway, which then went on to cause mitochondrial dysfunction and metabolic disorders.
The development of this model will open new avenues for scientific discovery in the study of mitochondria and multiple diseases.
More information: Haruna Tani et al, Aberrant RNA processing contributes to the pathogenesis of mitochondrial diseases in trans-mitochondrial mouse model carrying mitochondrial tRNALeu(UUR) with a pathogenic A2748G mutation, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac699
Journal information: Nucleic Acids Research
Provided by University of Tsukuba