June 29, 2022 feature
Microporous polymer membranes for light-gated ion transport
In a new report now published in Science Advances, Zongyao Zhou and a team of scientists in chemical engineering and physical science and engineering at the King Abdullah University of Science and Technology in Saudi Arabia developed an artificial light-gated ion channel membrane using conjugated microporous polymers. The team was inspired by light-gated ion channels in cell membranes that play an important role in many biological activities to precisely regulate the membrane pore size and thickness at the molecular level via bottom-up design and electropolymerization methods. The process led to reversible "on/off" light control for light-gated ion transport across the membrane to deliver hydrogen, potassium, sodium, lithium, calcium, magnesium and aluminum ions.
Light-gated membranes for ion transport
Light-gated ion channels can regulate the transport of ions in living cells to adjust electrical excitability, calcium influx, and other crucial cellular processes. At present, channelrhodopsins are the first and only class of light-gated ion channels identified in biology, and they have received much attention in recent years. The direct use of light-gated channelrhodopsins are limited by the generally minimal chemical and physical stability of the proteins in external environments. Researchers have therefore conducted extensive studies to develop artificial light-gated ion channels for applications across neurobiology, bioelectronics and waste purification.
Artificial light-gated ion channels can be produced in the lab by modifying nanopores with light-responsive functional groups. Conjugated microporous polymers (CMPs) provide a unique class of porous organic materials, as shown in previous work. In this work, Zhou et al synthesized a de novo rigid flexible azobenzene containing monomer (azo-CMP) to achieve the expected light-gated response. The team light-gated structurally well-defined elementary micropores and interconnected them to form smart ion channels in the azo-CMP membrane. The setup is best suited to facilitate photo-switching mechanisms to successfully achieve "on-off-on" photoisomerization for well-regulated ion transport.
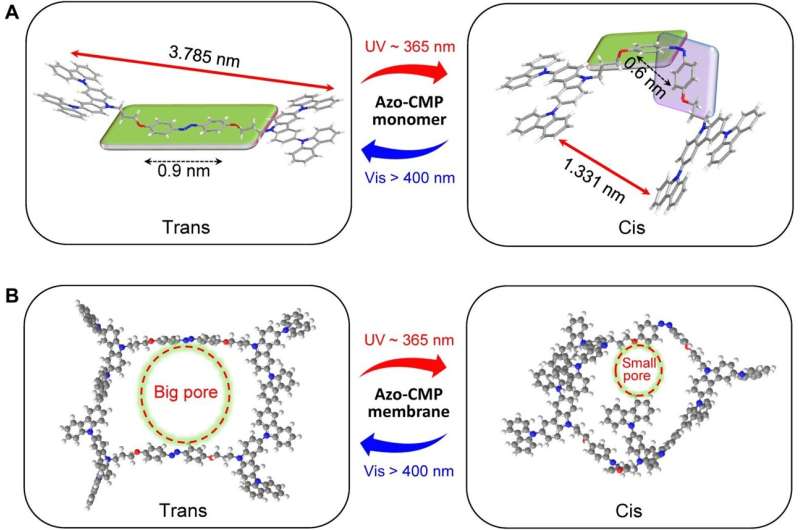
The azo-CMP monomer maintained a butterfly wing–like structure with azobenzene as the light-switchable hinge of the wing and the alkyl chain as a soft linker to bridge the hinge and electroactive carbazole scaffold. The team designed the length of the soft linker to increase the net distance and provided sufficient space for photoisomerization of the azobenzene moiety, which they analyzed via molecular simulations. During the experiments, the monomer showed rapid and reversible photoisomerization by changing the wavelength of irradiation.
Developing the azo-CMP membranes
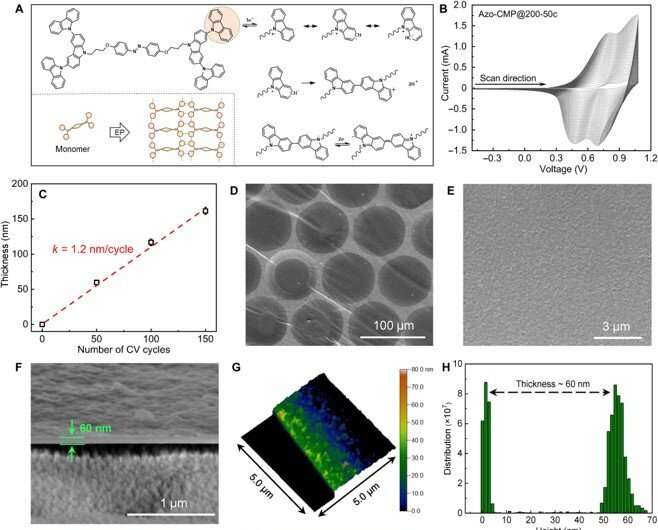
The scientists developed the azo-CMP membranes via electropolymerization in a three-cathode electrochemical cell. They optimized the reaction conditions for smooth and defect-free azo-CMP membranes and observed the resulting chemical structure via Fourier transform infrared spectroscopy. The outcomes confirmed the polymerization of carbazoles and the existence of azobenzene units in the membranes. The team modified the surface hydrophilicity and the appearance of membranes by modifying the synthetic parameters to create a tough and non-uniform membrane surface with many micro- and nano-structures.
Photoisomerization of the membrane
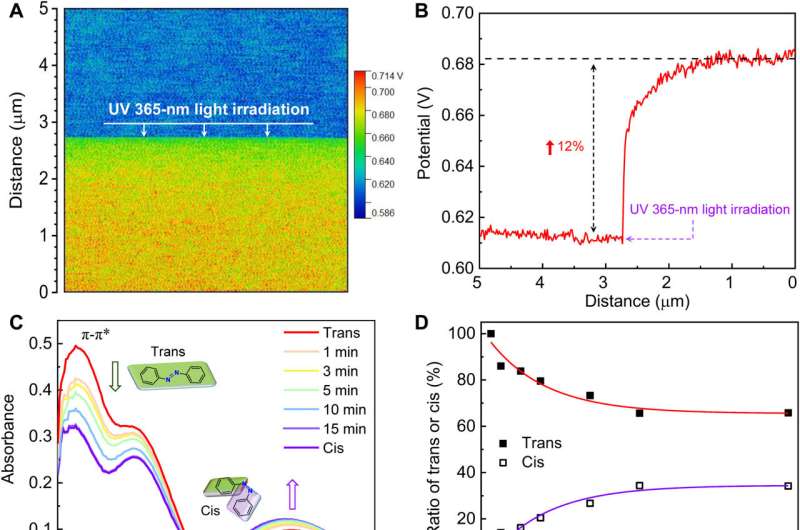
Photoisomerization can lead to structural changes in molecules and geometrical changes in the ion channels. Such structural changes can lead to a surface potential difference of the azo-CMP membranes, which Zhou et al observed by using real-time Kelvin probe force microscopy. The team recorded the changing surface potential of transmembranes after UV irradiation. The UV-Vis spectroscopy further substantiated isomerization of the membranes to indicate rapid and stable photo-responsive trans-cis-trans isomerization of azo-CMP membranes. The team used additional experiments to show changes in channel size of the membranes in trans and cis states through nitrogen adsorption isotherm measurements, followed by molecular dynamics simulations to reveal changes in the channel size for distinct ion permeability and selectivity.
-
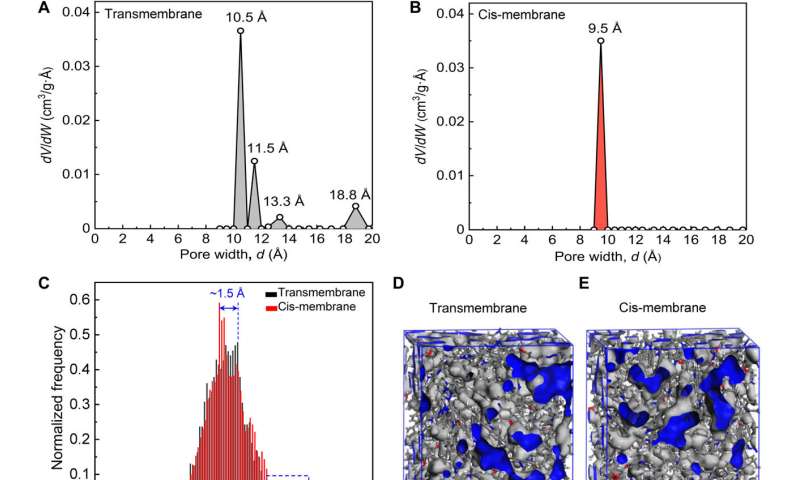
Pore-size distribution of azo-CMP@200-50c membrane. (A) The membrane in trans and (B) cis states. (C) Simulated pore-size distribution of the membrane in trans and cis states. A 3D view of the membrane in (D) trans and (E) cis states (free volume in gray and Connolly surface in blue). Credit: Science Advances (2022). DOI: 10.1126/sciadv.abo2929 -
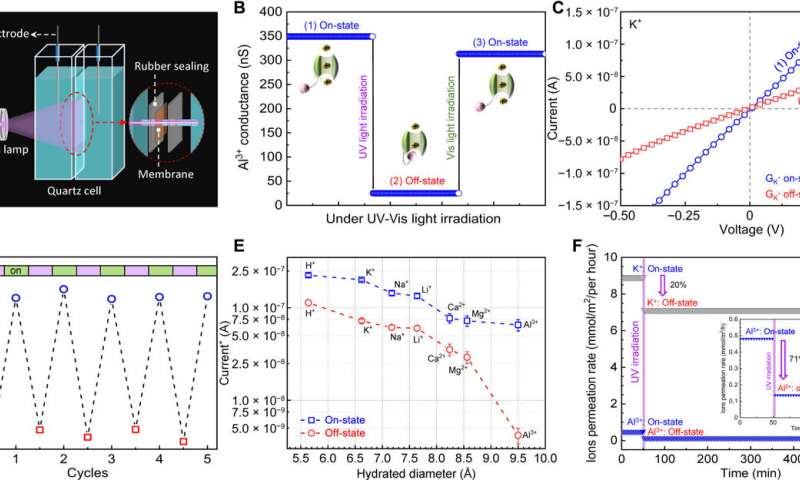
Light-gated ion transport of the membranes azo-CMP@200-50c. (A) Schematic diagram of the setup for the tests under electric field. (B) Al3+ conductance changes under alternating UV light and vis-light irradiation calculated on the basis of the data in fig. S28. The insets show the illustration of the controllable ion transport in the ion channels with on and off states. (C) I-V curves of the membranes recorded in 10 mM KCl solution during the trans-to-cis isomerization under UV light. (D) K+-relative conductance changes in successive cycles under alternating UV light and vis-light irradiation. The relative conductance is derived from comparing the conductance of K+ to that of deionized water (fig. S29). (E) Current of common ions recorded in on-state and off-state of the membrane under a voltage of 0.5 V. Note: The current in (E) was normalized by the number of the ion charges based on the data in figs. S28 and S30. (F) K+ and Al3+ permeation rate tested under concentration-driven ion permeation process. The inset shows the details of Al3+ permeation rate. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abo2929
Proof-of-concept: Light-gated ion transport of the membranes
The scientists studied the performance of light-gated ion channel membranes for controlled ion transport using electrically driven ion permeation tests in a lab quartz cell with two chambers. They filled the two chambers with similar concentrations of salt solution and measured ion transport via current-voltage characteristics of azo-CMP membranes in the trans and cis form.
They noted the dynamics of the membrane current/ion conductance in the "on-state" as well as decreased conductance upon UV irradiation to indicate a reduced ion transport state that could be restored via irradiation with visible light to regulate ion transport across smart channel membranes. The outcomes highlighted the scope of the of the light-gated ion channel membranes for pharmaceutical applications and smart dialysis.
Outlook
In this way, Zongyao Zhou and colleagues were inspired by naturally occurring channelrhodopsins to create reversible and recyclable artificial light-gated ion channel membranes. They designed azobenzene-containing conjugated microporous (CMP)-monomers at the molecular level by introducing a light-switchable azobenzene core unit a soft alkyl chain and rigid electroactive carbazoles. The chemistry of the membrane channels delivered a highly effective trans-to-cis photoisomerization response to regulate ion transport remotely and dynamically. The product is significantly important to the separation industry, including nanoscale molecule memory applications, smart drug release and photoresponsive chemosensors.
More information: Zongyao Zhou et al, Conjugated microporous polymer membranes for light-gated ion transport, Science Advances (2022). DOI: 10.1126/sciadv.abo2929
Farhan Mohammad et al, Optogenetic inhibition of behavior with anion channelrhodopsins, Nature Methods (2017). DOI: 10.1038/nmeth.4148
Journal information: Nature Methods , Science Advances
© 2022 Science X Network