Honeycomb structure with oxygen-poor pores found in oxide scale on small lead-based reactor materials

A research team led by Jiang Zhizhong from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences (CAS) has found a honeycomb structure in localized regions at the top of the magnetite layer on martensitic steel and analyzed its formation reason and process.
Small nuclear reactors especially lead-cooled fast reactors with lead-bismuth eutectic alloy (LBE) as the main coolant, have good application prospects in marine power, regional power supply, seawater desalination and other fields. However, homogeneous oxidation corrosion or dissolution corrosion may occur in steel materials, and LBE infiltration and pitting corrosion may also occur in local areas, due to the high solubility of ferrum, chromium and nickel in LBE at high temperature. Pitting corrosion is one of the most destructive corrosion forms, which will seriously affect the long-term safe service of small lead-cooled fast reactors.
"The honeycomb structure is composed of many oxygen-poor pores and surrounding net-like branches," said Luo Lin, member of the research team. "Low oxygen concentration promotes the formation of honeycomb structures in a shorter time and in more areas."
Moreover, pore size and oxygen-poor area increased with corrosion time. At 2,000 hours, the oxygen-poor zone extended to the magnetite layer and spinel layer below the pores, resulting in more oxygen vacancies in the above layers.
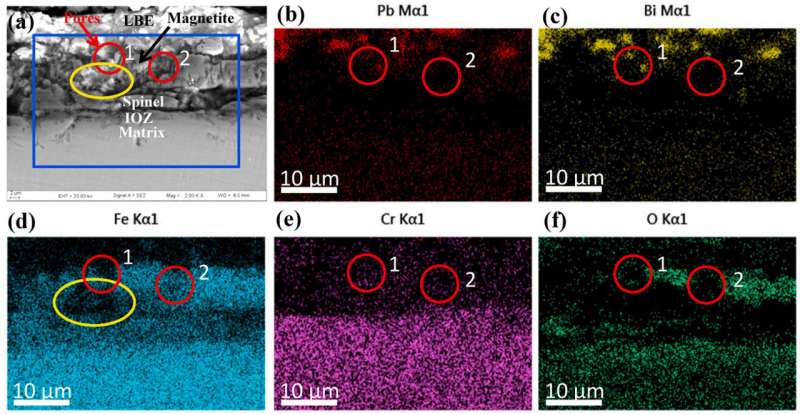
"The honeycomb structure may become a nucleation point of pitting corrosion and promote the development of pitting corrosion," said Luo.
This study shows the presence of honeycomb structure means that the low dissolved oxygen concentration in LBE is low, which requires optimal design of oxygen control system.
The research was published in Corrosion Science.
More information: Lin Luo et al, Honeycomb structure with oxygen-poor pores at the top of magnetite layer on a martensitic steel CLAM exposed to lead-bismuth eutectic at 500 ºC, Corrosion Science (2022). DOI: 10.1016/j.corsci.2022.110410
Provided by Chinese Academy of Sciences