Novel macrophage-mediated mechanism promotes peritoneal metastasis of ovarian cancer
A research team at the School of Biological Sciences, the University of Hong Kong (HKU), has revealed novel cellular and molecular interactions between cancer cells and tumor-associated macrophages that promote peritoneal metastasis of ovarian cancer. These findings provide important insights into the therapeutic strategy of ovarian cancer and are now published in Advanced Science, an interdisciplinary open-access journal.
Ovarian cancer is the leading cause of deaths among all gynecological cancers. More than 70% of patients are diagnosed at an advanced stage with metastatic diseases. Peritoneal metastasis is very difficult to treat due to tumor heterogeneity and the dynamic interactions of cancer cells with the tumor microenvironment. The lack of suitable experimental models has been one significant obstacle to study the cellular and molecular mechanisms of this critical process, and the distinct interactions among different cancer cell subclones and tumor microenvironment are largely unknown using traditional bulk measurement.
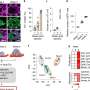
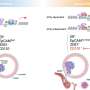
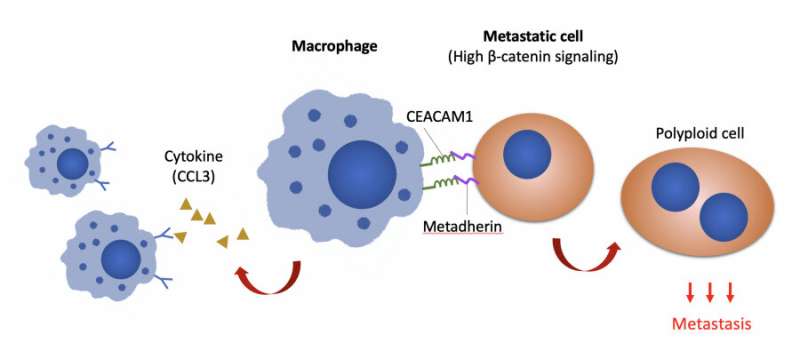
Key findings: In metastatic ovarian cancer cells, Wnt/b-catenin signaling upregulates the expression of metadherin, which communicates with macrophages through CEACAM1, a carcinoembryonic antigen expressed by macrophages, suggesting that blockade of macrophage-tumor communications (by inhibiting either metadherin or CEACAM1) could greatly reduce peritoneal metastasis.
Based on tumor heterogeneity, the research team has previously established an isogenic model that mimics spontaneous ovarian cancer metastasis. Using this model, an upregulation of Wnt/b-catenin signaling was found in the metastatic cells by gene profiling and bioinformatic analyses. Wnt/b-catenin signaling is known to play critical roles in embryonic development, tissue homeostasis and cancer development, since its upregulation increases oncogene expression and facilitates cancer metastasis.
Macrophages play key roles in both the innate and adaptive immunity to orchestrate the concerted immune responses and are the most abundant immune cells in the ovarian cancer tumor microenvironment. Observation of cellular behaviors using single-cell time-lapse microscopy reveals that in the presence of macrophages, a subset of the metastatic cells shows selective advantage of becoming polyploidy, a phenotype that duplicates entire genomes, could promote tumor aggressiveness and therapeutic resistance. On the other hand, the metastatic cells polarize macrophages to a tumor-associated phenotype that reinforces the polyploid phenotype. Further molecular analyses suggest that b-catenin signaling upregulates cancer cell surface metadherin, which communicates through CEACAM1 expressed by macrophages.
The clinical relevance of these scientific findings were further validated by tumor xenografts in mice and patients' clinical samples. Blocking macrophage-tumor communications via the inhibition of metadherin or CEACAM1 greatly reduced peritoneal metastasis in humanized mouse models that have human immune cells. Since metadherin and CEACAM1 are accessible on the outer surface of cells, they represent highly suitable candidates for tumor cell tracking and clinical targeting.
The team has made a key discovery of a potential driving mechanism for cancer cell polyploidy and genomic instability, which is initiated through direct interaction with macrophages. Targeting components of the molecular cascade identified in the study holds great therapeutic potential to disrupt polyploidization of the cancer subclones that drive metastasis.
"Our findings are intriguing because few factors that regulate cancer polyploidy have been identified to date, and we have also provided a mechanistic rationale for targeting b-catenin or its downstream signaling molecules to decrease peritoneal dissemination associated with poor prognosis," said Professor Alice Wong, Director (Interim) of the School of Biological Sciences, who led the research. The team plans to explore in more detail the signaling mechanisms that drive polyploidy in the metastatic cells, as this would greatly enhance the understanding of the genomically unstable disease.
More information: Sally K. Y. To et al, A Selective β −Catenin‐Metadherin/CEACAM1‐CCL3 Axis Mediates Metastatic Heterogeneity upon Tumor–Macrophage Interaction, Advanced Science (2022). DOI: 10.1002/advs.202103230
Journal information: Advanced Science
Provided by The University of Hong Kong