How the body's delivery system could lead to targeted therapeutics

In a post-pandemic world, we are used to sending and receiving parcels, getting groceries and items delivered to our door, sending messages and items to loved ones we cannot see. We've become accustomed to sending and receiving many tiny packages, but did you know that the best package system in the world can be found in our own bodies?
Dan Lark, Ph.D., assistant professor in the Colorado State University Department of Health and Exercise Science and principal investigator for the Extracellular Regulation of Metabolism Laboratory, is working with Zackary Valenti, a doctoral student in the department's Human Bioenergetics Ph.D. program, to study the key ways that cells communicate with each other.
In every human body, cells send and receive items constantly, accepting necessary nutrients and sending away waste by using extracellular vesicles, little capsules made of a lipid bilayer that buds off the first cell and travels to the accepting cell, which accepts it and reabsorbs the package of goods sent from the other cells. Almost all cells can create extracellular vesicles, and there are a variety of ways our bodies use these cells to communicate, replenish nutrients, or eliminate waste, as well as play key roles in processes for immune responses and coagulation, among other cellular responses.
Although we do know that extracellular vesicles play key roles in cell communication and many important body processes, there are still many questions about how and when extracellular vesicles operate, which could be critical biomarkers for disease. These questions are exactly what Lark, Valenti, and the research team at the Extracellular Regulation of Metabolism Laboratory want to answer.
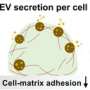
Valenti, along with a team of researchers, looked at how these extracellular vesicles are secreted by different tissue types, specifically skeletal muscle tissue and white adipose tissue, to see if these vesicles can be detected in the blood, an area of the field that has received minimal research in the past. Funded by the American Heart Association, the work aimed to see if the skeletal tissue naturally secreted more vesicles than the adipose tissue and aimed to define the origin and abundance of these vesicles so that they could be used as biomarkers and potentially lead to engineered therapies for patients.
Vesicles could be the key to the future of targeted medicine
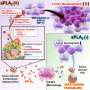
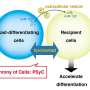
Just like the postal system, vesicles in your body are little packages, delivering RNA, DNA, lipids, proteins, and other cellular materials in between cells, both near and far to the cell the vesicle originated from, traveling through biofluids in the body to reach their destination.
Vesicles are known to play key roles in immune and repair responses, so researchers are interested in their abilities, as they could be a potential way to deliver future targeted treatments from cell to cell within the body, carrying medicine or engineered treatments to very specific targeted regions, or naturally rerouting existing vesicles to trigger natural immune responses when people's bodies are not producing the correct responses on their own.
The potential of this field for therapies is great, so researchers are very interested in determining how and why vesicles work and communicate, and when and how they are made, so that we can better track and predict them as both biomarkers to alert to possible injury and disease, as well as how they can be used in conjunction with existing therapies, or even lead to the creation of new, targeted therapies.
Lark and his research team have a particular interest in learning how extracellular vesicles work, and how to harness them for future therapies.
"Our laboratory is interested in understanding how cells, tissues, and organs communicate," says Lark. "We study small cell-derived particles called extracellular vesicles, which can be released from any cell in the body and have been implicated in cancer, metabolic, and cardiovascular diseases. We study how, when, and why EVs are released from skeletal muscle during exercise or in response to different diets. Our long-term goal is to identify EV-based therapies to prevent and/or treat metabolic disease."
Lark, Valenti, and others are co-authors of a new paper on the secretions of extra-cellular vesicles published in the American Journal of Physiology–Cell Physiology, released February 2022.
Shining a light on vesicle production
In order to be able to use these biological tools for the future of medicine, scientists and researchers are aiming to better understand how they operate and fill the gaps in our understanding of how they work naturally before looking at ways to use them for treatments.
"There are a number of factors that dictate how a given cell type will contribute to circulating extracellular vesicle (EV) abundance, including tissue mass, tissue EV secretion capacity, EV access to circulation, and EV clearance," explained Valenti and his research team in their recently released paper, "Extracellular vesicle secretion in tissue-dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo."
"None of these factors have been well defined, emphasizing the need for targeted approaches and novel methodologies," he said. "Skeletal muscle tissue and white adipose tissue are, by mass, two of the largest tissues in the body and would therefore be predicted to be major contributors to circulating EV abundance."
By studying the levels of EVs secreted from both tissues, and fluorescently labeling EVs from muscle, they were able to determine both the levels and origins of EVs being excreted from the different tissues, allowing the team to begin to set a foundation for future research. Future studies can now examine whether skeletal muscle releases EVs in response to natural stresses, such as exercise, and their influence on other organ systems.
More information: Andrea L. Estrada et al, Extracellular vesicle secretion is tissue-dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo, American Journal of Physiology–Cell Physiology (2021). DOI: 10.1152/ajpcell.00580.2020
Provided by Colorado State University