Ag3PO4 catalyst facilitates propylene oxide electrooxidation
There is a great need for the green production of propylene oxide (PO) due to its high industrial value. The electrooxidation of propylene into PO has aroused the interest of scientists because the process can be conducted under room temperature conditions and discharges no hazardous substance.
Based on the previously developed Ag electrode, which suffered from poor activity, a group led by Prof. Geng Zhigang from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences developed a catalyst composed of Ag3PO4 cubes with (100) facets. The catalyst displayed both high selectivity and high activity. The result was published in Nature Communications.
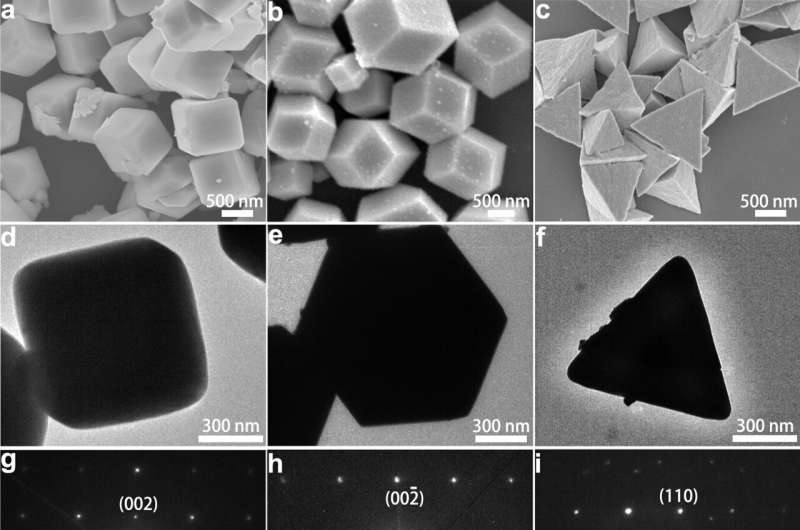
Researchers synthesized Ag3PO4 crystals with different facets and studied their catalytic performance in a three-compartment electrochemical cell. 1H nuclear magnetic resonance measurements revealed that Ag3PO4 cubes with (100) facets displayed a PO selectivity as high as 80%, while other Ag3PO4 samples with different structures displayed low PO selectivity. Compared with commercial Ag3PO4 without structural modification, Ag3PO4 cubes with (100) facets in this work displayed 10 times higher partial current densities of PO (jPO) normalized by electrochemical surface area (ECSA),demonstrating superior catalytic activity.
Density functional theory (DFT) calculations were also conducted to understand the reaction mechanism. The free energy diagram suggested that the reaction was likely preceded in a OH-related pathway, where *OH free radical participated in the reaction.
In the OH-related pathway, the formation of PrOH* free radical is the rate-determining step (RDS). The RDS had the lowest energy barrier on (100) facets of Ag3PO4. Moreover, researchers discovered from the Bader charge analysis that (100) facets had the strongest polarization of propylene, facilitating the breaking of π bonding and C-O bond formation. Taking these evidences into consideration, the superior catalytic activity of (100) facets of Ag3PO4 can finally be explained.
This work offered an effective PO electrocatalyst and deepened the understanding of the effect of crystal facets in catalysis.
More information: Jingwen Ke et al, Facet-dependent electrooxidation of propylene into propylene oxide over Ag3PO4 crystals, Nature Communications (2022). DOI: 10.1038/s41467-022-28516-0
Journal information: Nature Communications
Provided by University of Science and Technology of China