March 4, 2022 report
Tracing the diffusion of carbon isotopes using atomic-scale vibrational spectroscopy
A team of researchers affiliated with multiple institutions in Japan has developed a technique for tracing the diffusion of carbon isotopes using atomic-scale vibrational spectroscopy. In their paper published in the journal Nature, the group describes how they used isotopic imaging of 12C carbon atoms embedded in 13C graphene to monitor self-diffusion. Jordan Hachtel, with Oak Ridge National Laboratory, has published an outline of recent research involving attempts to detect isotopes with high spatial resolution and the work done by the team in Japan in the same journal issue.
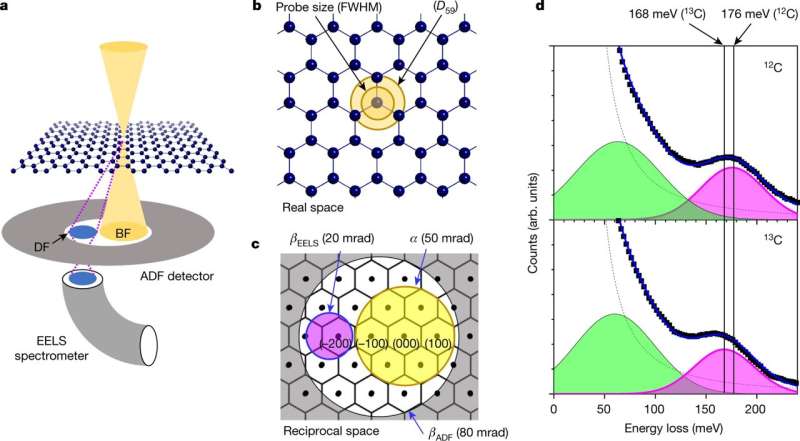
As Hachtel notes, isotopes are used in a wide variety of applications but it is still difficult to distinguish one from another at the atomic level. Doing so requires being able to see the differences in behavior based on their masses (isotopes of the same element have the same number of protons but different numbers of neutrons, which account for differences in mass). A transmission microscope can be used to capture images of the electrostatic potential of individual atoms but does not allow detecting differences in mass. Also, electron energy loss spectroscopy involves irradiating samples with electrons to measure energy loss due to collisions within a sample—an approach that involves vibrational spectroscopy. In this new effort, the researchers used both approaches to allow them to observe individual isotopes of carbon.
Their work involved preparing two graphene samples, one with atom masses of 12, the other 13. Each was measured for dark-field energy loss to ascertain peak characteristics. Next, the researchers attempted to fill cracks in one of the samples with material from the other by heating the samples to 650° C while also firing electrons at it. Doing so resulted in decomposition of the hydrocarbons that were surrounding the samples, allowing the crack to be filled. Next, the temperature was lowered to 500° C allowing measurement of the vibrational electronic spectra to prove the crack was filled. The team then repeated the exercise but maintained the higher temperature for an additional two hours. They then conducted vibrational spectral analysis to show that the 12C carbon atoms were distributed throughout the sample, allowing them to observe the process of diffusion of the isotopes of a single element.
More information: Ryosuke Senga et al, Imaging of isotope diffusion using atomic-scale vibrational spectroscopy, Nature (2022). DOI: 10.1038/s41586-022-04405-w
Jordan A. Hachtel, Isotopes tracked on a sub-nanometre scale using electron spectroscopy, Nature (2022). DOI: 10.1038/d41586-022-00545-1
Journal information: Nature
© 2022 Science X Network