A novel nanoplatform for delivering drugs into lymphocytes
T cells, also known as lymphocytes, have important roles in various immune reactions. However, there are only a few reports on delivery systems into T cells.
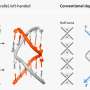
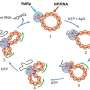
Associate Professor Chie Kojima and her co-workers from Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University (OPU), collaborating with Professor Ikuo Fujii and Ikuhiko Nakase from Department of Biological Science, Graduate School of Science, OPU, have performed an experimental study constructing a pH-sensitive delivery system into T cells and their subsets by using carboxy-terminal dendrimers (highly ordered, branched polymeric molecules) bearing phenylalanine (Phe) and hydrophobic acid anhydride (cyclohexanedicarboxylic anhydride, CHex), such as PAMAM-CHex-Phe and PAMAM-Phe-CHex. These dendrimers showed a higher association with splenocyte-derived T cells, which suggests that the hydrophobic effect significantly influences the association of dendrimers with immune cells.
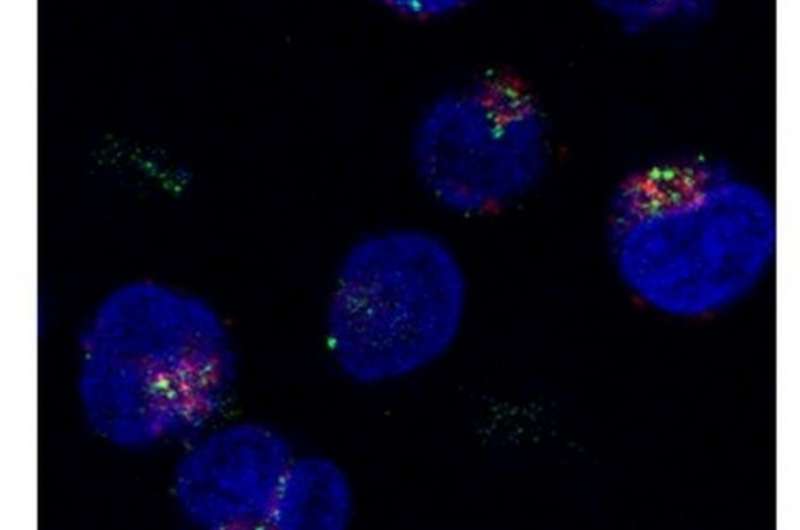
The T cell association of these dendrimers was examined at different pH and temperatures using fluorescence-activated cell sorting, where murine splenocytes stained with an anti-CD3 antibody were used. Along with this, the association of PAMAM-CHex-Phe and PAMAM-Phe-CHex with some culture cell lines and T cell subsets, such as CD4-positive helper T cells (CD3+CD4+), CD8- positive killer T cells (CD3+CD8+) and activated T cells (CD3+CD69+) was also examined. In order to confirm the internalization of these dendrimers into T cells, Assoc. Prof. Kojima and her co-workers used confocal microscopic imaging to observe the intracellular distribution of PAMAM-CHex-Phe and PAMAM-Phe-CHex.
Assoc. Prof. Kojima says that "although T cells play important roles in various immune reactions, there are only a few reports on delivery systems into T cells. In this study, we applied the Phe-modified dendrimers to a pH-sensitive drug delivery system into T cells. Dendrimers with different amino acids and acid anhydrides were synthesized, and their pH-responsive association with T cells and their subsets was investigated."
This experimental study by Assoc. Prof. Kojima and her co-workers has successfully presented the findings in terms of a) Synthesis and pH sensitivity of the carboxy-terminal dendrimers bearing Phe; b) pH-responsive association of the carboxy-terminal dendrimers bearing Phe with T cells and T cell subsets including activated T cells; c) Internalization of PAMAM-Phe-CHex and PAMAM-CHex-Phe into T cells.
She concludes that their "results showed that Phe- and CHex-modified dendrimers have a delivery potential to T cells and their subsets. This will play a key role in cancer immunotherapy."
Activation of lymph node-resident helper T cells and killer T cells as well as suppression of regulatory T cells localized in tumor tissues is necessary in cancer immunotherapy. Hence, based on the pH-responsive properties, the findings (dendrimers) obtained by Assoc. Prof. Kojima and her co-workers are important for the development of nanoplatforms for direct delivery to T cells to control the functions of T cells, which are useful for cancer immunotherapy.
The article was published in the Journal of Materials Chemistry B.
More information: Hiroya Shiba et al, Carboxy-terminal dendrimers with phenylalanine for a pH-sensitive delivery system into immune cells including T cells, Journal of Materials Chemistry B (2021). DOI: 10.1039/D1TB01980E
Provided by Osaka Prefecture University