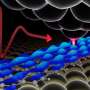
Engineered particles efficiently deliver gene editing proteins to cells in mice
Gene editing approaches promise to treat a range of diseases, but delivering editing agents to cells in animal models and humans safely and efficiently has proven challenging. Now, researchers led by a team at the Broad Institute of MIT and Harvard have developed a way to get gene editing proteins inside cells in animal models with high enough efficiency to show therapeutic benefit.
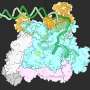
In new work published in Cell, the team shows how they have engineered virus-like particles to deliver base editors—proteins that make programmable single-letter changes in DNA—and CRISPR-Cas9 nuclease, a protein that cuts DNA at targeted sites in the genome. In collaboration with research teams led by Krzysztof Palczewski at the University of California, Irvine, and Kiran Musunuru at the Perelman School of Medicine at the University of Pennsylvania, the team used their particles, called engineered virus-like particles (eVLPs), to disable a gene in mice that can be associated with high cholesterol levels, and partially restored visual function to mice harboring a mutation that causes genetic blindness.
Scientists have long studied virus-like particles as potential drug delivery vehicles. Virus-like particles (VLPs) are small structures of viral proteins that carry molecular cargo, but do not contain viral genetic material and do not cause infection. Because VLPs lack viral genetic material, they may be safer than other delivery methods that use actual viruses, which can insert their genetic material into the cell's genome and potentially cause cancer.
The Broad team identified several features of VLPs that limit their delivery efficiency, and engineered changes to the structure of the particles to overcome these bottlenecks. They say the resulting eVLPs are the first virus-like particles to deliver therapeutic levels of gene editing proteins to a variety of tissues in adult animals.
In their study, the team did not detect any off-target editing when they used eVLPs to deliver the gene editing machinery as protein, but did when the editors were delivered as DNA. Their observations confirm previous research showing the benefits of using protein forms of gene editors and shows that eVLPs can safely deliver them. The scientists add that their eVLPs could potentially be used not just for gene editing, but also to deliver other therapeutic proteins.
"In vivo delivery has proven to be a recurring challenge despite the fact that it's an critically important aspect of any future in which gene editing plays a major role," said David Liu, senior author of the study and Richard Merkin Professor and director of the Merkin Institute of Transformative Technologies in Healthcare at the Broad. Liu is also a Howard Hughes Medical Institute investigator and a professor at Harvard University.
"VLPs have always been one of the most attractive delivery technologies but have suffered from inefficient in vivo protein delivery," Liu said. "By rationally engineering molecular solutions to address specific challenges in the VLP delivery process we developed eVLPs that greatly increased delivery potency in cultured cells, and also enabled efficient delivery in animals."
Delivery bottlenecks
For decades, virus-like particles have been of interest to researchers because they behave like viruses in that they can enter cells and deliver cargo such as therapeutic proteins. Researchers can influence the ultimate destination of VLPs in the body—the liver or neurons, for instance—by using different molecules on the surface of the particles.
To take advantage of these features and improve delivery, Liu's team systematically engineered different parts of the VLP architecture to optimize several critical steps—how VLPs are produced, how cargo is packaged into VLPs, and how the cargo is released and distributed within cells.
The final version of their eVLP packaged 16 times more cargo proteins than their previous designs, and enabled an eight- to 26-fold increase in editing efficiency in cells and animals. As the team had hypothesized, they saw little evidence of editing at undesired locations, and no incorporation of viral DNA into cells treated with eVLPs.
"Because eVLPs offer robust on-target editing and minimize off-target editing, we hope they'll serve as a safer method for delivering gene editing agents in vivo," said Aditya Raguram, co-first author of the study and a Ph.D. student in Liu's lab.
eVLPs in action
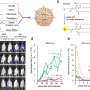
The team used their optimized eVLP system to correct mutations in a range of mouse and human cells, observing 95 percent editing efficiency in some cases.
The scientists then used eVLPs to deliver base editors to the liver in mice, where they efficiently edited Pcsk9, a gene that, when mutated, can dramatically lower "bad" cholesterol levels in the blood, decreasing heart disease risk for some patients. The researchers showed that a single injection of eVLPs programmed to install such a mutation resulted in an average of 63 percent editing of Pcsk9 and a 78 percent drop in Pcsk9 protein levels. The team says they expect these outcomes would substantially reduce an individual's risk of coronary heart disease.
The researchers also used a single eVLP injection to restore visual function in mice bearing a mutation for blindness. They corrected a mutation in the Rpe65 gene with comparable editing efficiency to other base editing delivery techniques but with less off-target editing and risk of viral DNA integration.
The team also injected eVLPs directly into the brain in mice and observed around 50 percent editing efficiency in cells exposed to the eVLPs. Future efforts will focus on improving eVLP distribution throughout the brain, but the results show promise for delivering gene editing agents to an organ that is notoriously difficult to target.
"eVLPs combine advantages of both viral and non-viral delivery systems," said Samagya Banskota, co-first author of the study and a postdoctoral fellow in the Liu lab. "They're also programmable and relatively easy to produce, which makes them promising tools for protein delivery. We look forward to the scientific community adopting our eVLPs and using them to improve therapeutic macromolecule delivery for patients."
Liu's group is now expanding the range of organs and cell types that eVLPs can target in animals. They will also continue to characterize eVLPs to better predict and mitigate any unwanted immune responses the particles may produce.
"Now that we know some of the key eVLP bottlenecks and how we can address them, even if we had to develop a new eVLP for an unusual type of protein cargo, we could probably do so much more efficiently," Liu said, noting that their eVLP effort began in early 2018.
"There's so much need for a better way to deliver proteins into various tissues in animals and patients," Liu said. "We're hopeful that these eVLPs might be useful not just for the delivery of base editors, but also other therapeutically relevant proteins."
More information: Samagya Banskota et al, Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins, Cell (2022). DOI: 10.1016/j.cell.2021.12.021
Journal information: Cell
Provided by Broad Institute of MIT and Harvard