Localis-rex: A new tool for studying electrophile signaling
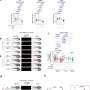
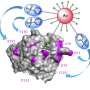
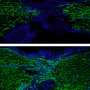
![Quantitative and comparative indexing of locale-specific native first responders. Also see SI Appendix, Figs. S1–S13. (A) Localis-rex setup for indicated subcellular locales. (Left) Mitochondrial outer membrane (MOM) vs. nuclear-targeted spatiotemporally controlled as well as dosage-controlled release of electrophilic signals, exemplified by alkyne-functionalized hydroxynonenal (HNE) (red dot). MOMLS, MOM-localization sequence. NLS, nuclear-localization sequence. Ht-PreHNE, Halo-targetable photocaged HNE(alkyne). (Right, Inset) A representative Localis-rex strategy (SI Appendix, Fig. S1A), coupled with the established target- and signal-specific pathway-interrogation method, T-REX (10, 23) (SI Appendix, Fig. S1B). (B) Validation of candidate first responders by T-REX (10, 23) in HEK293T cells expressing the respective Halo-TeV-Flag2-POI (POI, protein of interest; “TeV” designates a linker housing a TeV-protease-cleavable site, here and elsewhere) by Click-biotin pulldown assay followed by Western blot analysis using indicated antibodies. Cell lysates were subjected to Click coupling (with biotin-azide), protein precipitation, resolubilization, streptavidin enrichment, washing, and elution, followed by Western blot analysis. (C) Determination of fractional ligand occupancy on CDK9 following T-REX (10, 23, 49) in HEK293T cells expressing Halo-TeV-Flag2-CDK9 (against controls: light alone, Ht-PreHNE-probe alone; see SI Appendix, Fig. S1 B, Inset). (Left) Representative data from Click-biotin pulldown assay (49) followed by Western blot analysis using indicated antibodies. (Right, Inset) Quantitation. Hydroxynonenal occupancy was calculated as the ratio of [“Flag2-CDK9” precipitated post–TeV-mediated separation of Halo and CDK9 in T-REX–treated samples]:[“Halo-TeV-Flag2-CDK9” precipitated from light-protected and non–TeV-treated samples], which is equivalent to (hydroxynonenylated-CDK/total CDK9). (n = 3 biological replicates; error bars, SD; two-tailed t test was applied.). Credit: DOI: 10.1073/pnas.2120687119 Localis-rex: A new tool for studying electrophile signaling](https://scx1.b-cdn.net/csz/news/800a/2022/localis-rex-a-new-tool.jpg)
In general, an electrophile is a highly reactive compound that "seeks" to bond with atoms or other molecules that have an available electron pair. In the buzzing hive of activity that is the cell, electrophile signaling is a process that regulates the signaling properties of proteins.
Electrophile signaling is also directly related to drug-target discovery and in our understanding of drug mode of action. In fact, there is an entire field of pharmacology that aims to improve the efficacy of certain drugs by adding electrophilic "parts" to them.
Intriguingly, as the proteins move across the cell's subcellular compartments, they may encounter different reactive small-molecule metabolites, which are low-molecular weight compounds, typically involved in a biological process as a substrate or product. Some of these compounds are themselves electrophilic. Thus, it is interesting to ponder how a specific protein's "electrophile-sensing" properties may change across different parts of the cell.
In 2021, Aye's group developed an innovative chemical method to study the biological mechanisms of the electrophilic drug Tecfidera, which is used to treat multiple sclerosis. Now, the team has developed a new tool, named "Localis-rex," that allowed them to delve deeper into electrophile biology and address some unanswered questions in the field. The study is published in PNAS.
Localis-rex: Solving electrophile signaling mysteries
"Electrophile signaling is typically viewed in one of two ways," says Aye. "First, the random collision of small reactive molecules and reactive proteins that leads to a muddied response that may affect some cell signaling pathways. Second, the localized production of an electrophile labeling a protein due to proximity, leading to downstream signaling."
The problem is that proteins themselves can change their own sensing properties as they move through different areas of the cell; something that the field has given little thought to, according to Aye. "Even less thought has been invested into how the electrophile-modified state, itself significantly more stable and diffusive than the electrophile signals themselves, may signal across organelles," she adds.
The aim of Localis-rex was to explore all those questions by identifying metabolite "sensors" located in subcellular compartments –in this case the nucleus or the outer membrane of the mitochondrion. The method works by releasing a transient burst of an electrophilic compound in a specific subcellular compartment. There, the compound reveals (or not) potential reactive metabolites that sense and regulate a signaling protein.
Novel findings
"Using Localis-rex, we identified 32 locale-specific sensor proteins sensing a native lipid-carbonyl-based metabolite, namely, hydroxynonenal," says Aye. "Twenty percent of these proteins were not previously identified as being sensitive to electrophiles or covalent-drugs at all, while others were not even known to be sensitive specifically to hydroxynonenal, a quintessential lipid-derived electrophilic-metabolite." Hydroxynonenal is of particular interest to Aye's group, because they recently showed it to be a novel covalent fragment for kinase-isoform-specific drug development, an important field of pharmacology.
About forty percent of the sensor proteins that Localis-rex identified sensor electrophilic-metabolites in areas of the cell where these proteins normally neither localize nor are active. The researchers focused on CDK9, a protein that is involved in transcription, the process of encoding a gene from DNA to mRNA. Their work showed CDK9 is indeed a cytosol-specific sensor of electrophiles.
It is important to note here that transcription, which is as critical to life as it is studied, has never before been thought to be regulated by electrophiles and there are few advanced therapeutics targeting it.
"Localis-rex is likely to be most informative for rigorous identifying of the best electrophile-sensors," says Aye. "Our work has important ramifications for covalent drug design and also profiling of drug-sensitive cells with high spatiotemporal resolution, as well as establishing interesting and relevant means through which endogenous signaling electrophiles and reactive metabolites can impact biological signaling processes."
More information: Yi Zhao et al, Function-guided proximity mapping unveils electrophilic-metabolite sensing by proteins not present in their canonical locales, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2120687119
Journal information: Proceedings of the National Academy of Sciences
Provided by Ecole Polytechnique Federale de Lausanne