March 5, 2021 report
Using a radical to break C-F bonds one at a time
A team of researchers from the University of Science and Technology of China and the University of California has found a way to use radicals to break C-F bonds one at a time when working with trifluoroacetamides and acetates. In their paper published in the journal Science, the group describes how they found the right radical for such reactions and how their technique might be used in future applications.
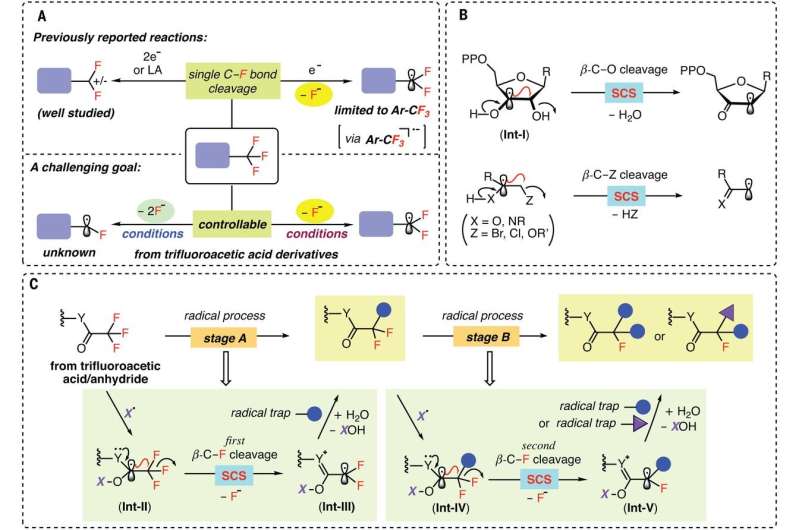
Chemists have been searching for ways to add fluorine to certain drugs because doing so helps their molecules move through cell membranes. The problem has been finding a way to swap out just one of the fluorine atoms in compounds such as trifluoromethyl to create mono- and/or difluorinated compounds. With current methods, when a reaction breaks the first C-F bond, the other two grow weaker, resulting in their removal, as well. In this new effort, the researchers have found a way to carry out such reactions without weakening secondary C-F bonds.
The researchers claim that the solution to the problem lies in finding the right radical—one that can target trifluoroacetate and trifluoroacetamide starting materials. After an extensive search, they found that the amino-boryl radical would target starting compounds as desired. The radical worked, they note, because the CF3 carbon molecule was strongly electron deficient. That meant that once the radical broke the first C-F bond, it was much less likely to do the same for the second or third C-F bonds. The end result was a reaction that stopped before all the fluorine bonds had been broken.
The team tested their radical along with an amino boran by swapping out a C-F bond for either a C-H bond or a C-C bond, while also adding other ingredients such as hydrogen to make a wide variety of mono- and difluorinated acetamides and acetates. They also used their radical to modify existing drug molecules to make them more effective in targeting specific tissue. They suggest their technique should prove useful in the development of new therapies for a host of ailments, ranging from lymphoma to endometriosis.
More information: Sequential C–F bond functionalizations of trifluoroacetamides and acetates via spin-center shifts, Science (2021). science.sciencemag.org/lookup/ … 1126/science.abg0781
Journal information: Science
© 2021 Science X Network