Collagen structures get the royal reveal
Collagen is the king of biological proteins, and now it has a SCEPTTr.
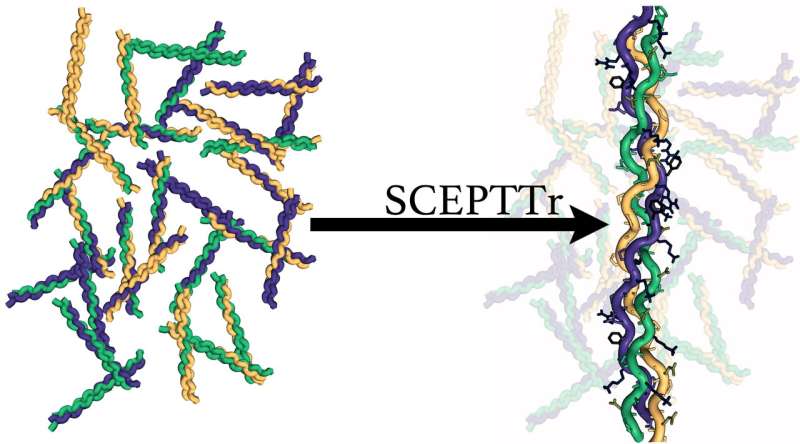
That's the handle of an algorithm developed by Rice University scientists who study natural and synthetic versions of collagen, which accounts for about a third of the body's proteins and forms the fibrous glue in skin, bones, muscles, tendons and ligaments.
The program—full name, Scoring function for Collagen-Emulating-Peptides' Temperature of Transition—accurately predicts the stability of collagen triple helices, the primary structure that forms fibrils.
The Rice team led by chemist and bioengineer Jeffrey Hartgerink and graduate student Douglas Walker tested SCEPTTr on 431 collagen triple helices from their own experiments and from literature and also used it to guide the design of a novel synthetic collagen helix.
The study in Nature Chemistry could help researchers better understand the critical protein's role in wound healing and cancer and could be used to design synthetic collagens, according to the researchers.
The paper significantly improves upon the lab's 2012 project to predict the stability of synthetic collagen structures. "One of its limitations it that it only understood a very narrow range of amino acids," Hartgerink said.
SCEPTTr expands that to the entire array of natural amino acids. That essentially makes it an Erector set for collagen design, potentially collapsing weeks of trial and error down to seconds on a desktop computer.
"The second major improvement is that while the earlier work couldn't tell you if a species of collagen would be stable at room temperature, Doug's program makes predictions about the melting temperature, the point at which a triple helix falls apart," Hartgerink said. "The great part about that is it's an easily testable value that extends to physiological temperatures as well.
"That's important to us, because if it falls apart when you put it inside a living organism, it's not particularly useful," he said.
SCEPTTr returns more than a single result for a given trio of sequences, detailing all possible combinations of the input peptides and providing a melting temperature for each.
"One of the most important things SCEPTTr does is look at all of the alternative structures that are possible and tells you how stable those are," Walker said. "It helps us understand what these complex mixtures are going to look like before you make them."
Walker brought a unique combination of talents to the project, spending half his time synthesizing collagen peptides and half programming SCEPTTr.
"When I was an undergraduate (studying chemistry at Idaho State University), I kept taking math classes because I really enjoyed it," he explained. "And it got to the point where I thought, 'Well, I'm one class away from a math minor.' And that happened to be a computer science course."
Despite reservations, Walker found he enjoyed programming. "Early on, I said, 'Hey, Doug, do you know anything about computers, because I've got a project for you,'" Hartgerink recalled. "I think the idea was that, if he didn't know about programming, he was going have to figure it out one way or another."
Walker said the program will be freely available online. "We want to make this a more accessible field, and SCEPTTr should make it easier for people to understand collagen," he said.
More information: Predicting the stability of homotrimeric and heterotrimeric collagen helices. Nat. Chem. (2021). doi.org/10.1038/s41557-020-00626-6
Journal information: Nature Chemistry
Provided by Rice University