Bioinspired strategies for the development of new drugs
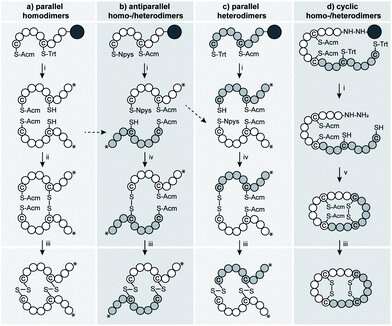
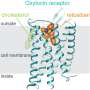
MedUni Vienna researchers led by Christian Gruber from the Institute of Pharmacology, with international collaborators, have shown that it is possible to dimerize the human neuropeptides oxytocin and vasopressin without forfeiting much of their efficacy. The researchers were inspired by a naturally occurring locust neuropeptide in the form of a dimer of two vasopressin-like molecules. "By making structural changes to the composition and orientation of the dimers, we managed to modify their pharmacological activity to achieve selective inhibition or activation of the human vasopressin V1a receptor," explains Christian Gruber from MedUni Vienna. Markus Muttenthaler from the University of Vienna adds: "Dimerization is a strategy that is often observed in Nature to optimize stability or mode of action. A good example of this is insulin, which is also a dimer of two different peptides."
600-million-year-old oxytocin-vasopressin signaling system
The researchers used a unique strategy for the discovery of new oxytocin and vasopressin molecules with different pharmacological properties, exploiting the advantages of the evolutionary similarities of the oxytocin-vasopressin signaling system that first evolved around 600 million years ago and is widely distributed in the animal kingdom. These new pharmacological probes provide new insights into receptor function and could also represent new therapeutic leads for several disorders.
"Our concept is as innovative as it is fascinating: You take an insect neuropeptide, study its structure and replicate this with minor chemical changes to obtain therapeutic leads for human diseases," explain Gruber and Muttenthaler. "It is equally important to make these new molecules available as research tools. Only by developing receptor-subtype-selective molecules is it possible to investigate the physiological relevance of these signaling systems."
Functions of the oxytocin-vasopressin signaling system studied
The researchers aim to explain the pharmacological and physiological relevance of this signaling system. "We have studied the function of this signaling system in ants and found that the oxytocin-vasopressin hormone system regulates foraging, physical activity and metabolism." The researchers therefore assume that oxytocin is not merely a "love hormone," but potentially also acts as an appetite suppressant—an application worth investigating.
More information: Zoltan Dekan et al. Nature-inspired dimerization as a strategy to modulate neuropeptide pharmacology exemplified with vasopressin and oxytocin, Chemical Science (2021). DOI: 10.1039/D0SC05501H
Journal information: Chemical Science
Provided by Medical University of Vienna