Recovery of ovarian function in infertile mammals lacking gonadotropin release
Gonadotropins are any hormones that are released from the anterior pituitary to stimulate the gonads, or sex glands, to carry out their reproductive functions. The gonadotropin-releasing hormone (GnRH) is therefore fundamental for mammalian reproduction. In a healthy reproductive system GnRH is produced by the brain in pulses. Reports suggest that at least 25% of ovarian disorders are due to dysfunction of the brain mechanism controlling the release of gonadotropins, which is a kind of reproductive disorder associated with the hypothalamus.
However, the source of the GnRH pulse generator has been a mystery for decades. New research carried out by a Japanese collaboration between the Graduate School of Bioagricultural Science, Nagoya University, and the National Institute of Physiological Sciences in Okazaki City, Japan, published in the Proceedings of the National Academy of Sciences, provides the first direct evidence that KNDy neurons generate the GnRH pulses, and that lack of these inhibits fertility. Using a rat model, the study also showed that rescuing just 20% of the KNDy neurons is sufficient to restart the GnRH and gonadotropin pulses and to maintain growth of ovarian follicles in the ovaries, thereby recovering fertility.
"Pulsatile GnRH release into the pituitary portal vein from the hypothalamus is fundamentally important for gonadal function in mammalian species, including humans," says Hiroko Tsukamura, a professor at Nagoya University and the corresponding author of the paper.
"GnRH enables stimulation of the release of gonadotropins, which in turn stimulate the growth of ovarian follicles only when GnRH is secreted in a pulsating manner at the appropriate frequency. Thus, GnRH pulse regimens are needed as therapies for infertile women as continuous GnRH treatment paradoxically inhibits gonadotropin release."
After the discovery of kisspeptin neurons in the brain as a dominant stimulator for GnRH release, circumstantial evidence had suggested that a subpopulation of these neurons, located in the hypothalamic arcuate nucleus (near the base of the hypothalamus), is responsible for GnRH pulses. However, no direct evidence was available that these KNDy neurons serve as the GnRH pulse generator. The acronym KNDy comes from the peptides (signaling molecules) kisspeptin, neurokinin-B (NKB) and dynorphin-A released by this part of the hypothalamus: for this reason, arcuate kisspeptin neurons are also known as KNDy neurons. The research by the Japanese team reported here has shown that NKB increases the frequency of GnRH pulses, while dynorphin-A reduces their frequency.
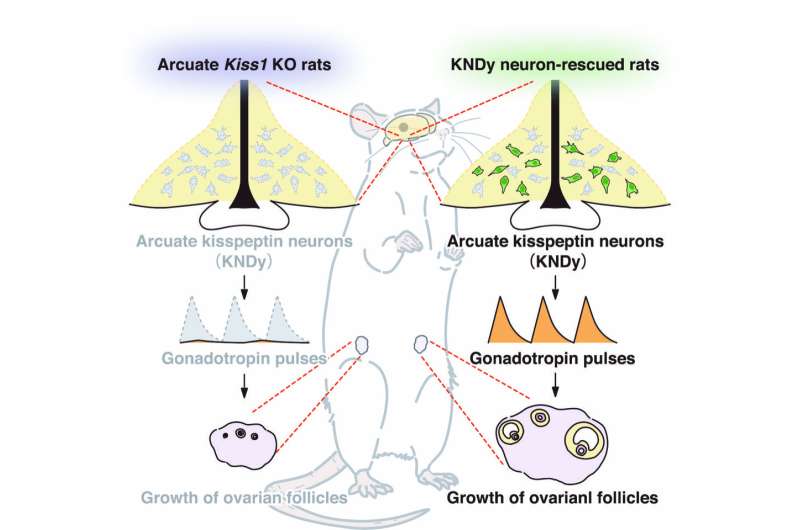
The research team used a congenitally infertile animal model (a rat) in which the kisspeptin gene (Kiss1) had been genetically deleted. The team rescued KNDy neurons by transfecting the Kiss1 gene into the rat's brain using a viral vector carrying the gene. The team noted that rescuing just 20% of the KNDy neurons (by forced expression of Kiss1 inside, but not outside, the NKB neurons) resulted in the release of pituitary gonadotropins, with concomitant growth of ovarian follicles up to pre-ovulatory size. Importantly, rescuing as little as 20% of the KNDy neurons was also enough to maintain ovarian function in female rats.
The results of the KNDy neuron-rescue experiment were confirmed in the second stage of the research by reversing the process in the first stage. This time, as the co-first-author, Ph.D. student Mayuko Nagae explains, "We prepared genetically modified rats in which the Kiss1 gene was sandwiched between a DNA sequence named loxP. This enabled us to delete the Kiss1 gene only in KNDy neurons by excising the DNA sequence between the two loxP sites by carefully injecting a viral vector carrying the gene-encoding Cre recombinase, an enzyme that targets loxP sites in the hypothalamic arcuate nucleus. The rest of the kisspeptin neurons were left unaltered. In this way, we engineered conditional brain-region-specific Kiss1 gene deletion in KNDy neurons."
Deleting more than 90% of the Kiss1 gene from the specific subpopulation of kisspeptin neurons resulted in complete suppression of pulsed release of gonadotropins.
Taken together, these findings provide the first direct evidence that KNDy neurons are the GnRH pulse generator and that rescuing just 20% of the KNDy neurons is sufficient to restart the GnRH and gonadotropin pulses and to maintain growth of ovarian follicles, thereby recovering fertility.
The finding provides a potential therapeutic approach for patients with hypothalamic reproductive disorders. In particular, since the NKB peptide agonist or dynorphin-A peptide antagonist enhances the GnRH pulse generation, long-term administration of these NKB agonist or dynorphin-A antagonist using a sustained-release drug could be useful in enhancing GnRH pulses in patients. This would replace pulsed infusion of GnRH by an attached pump. This methodology could also be applied to domestic animals, because mammalian species, including domestic animals, such as cattle, sheep, goats, and pigs, primates as well as rodent experimental models, have the same brain mechanism for regulating reproductive function via hypothalamic kisspeptin neurons.
"At last, we found direct evidence that KNDy neurons are the GnRH pulse generator," says Professor Tsukamura. "We have been seeking the source of the GnRH pulse generator—since long before the discovery of KNDy neurons—together with the late Dr. Kei-ichiro Maeda, of the University of Tokyo, the former Professor and PI of the Nagoya group, who suddenly passed away during this collaboration. We dedicate this study to him with gratitude for his leadership, supervision, and original ideas."
Professor Tsukamura and co-first-author Associate Professor Uenoyama think there is a lot more work to be done to find the molecular mechanism that controls KNDy neuronal, GnRH-pulse-generating activity. Nevertheless, they say, the present findings help illuminate the central mechanism underlying mammalian reproduction, and can be applied to the treatment of ovarian disorders in livestock as well as infertility in humans.
More information: Mayuko Nagae el al., "Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator," PNAS (2021). www.pnas.org/cgi/doi/10.1073/pnas.2009156118
Journal information: Proceedings of the National Academy of Sciences
Provided by Nagoya University