Enzymatic photocaging for the study of gene regulation through DNA methylation
The addition and removal of methyl groups on DNA plays an important role in gene regulation. In order to study these mechanisms more precisely, a German team has developed a new method by which specific methylation sites can be blocked and then unblocked at a precise time through irradiation with light (photocaging). As reported in the journal Angewandte Chemie, the required regent is produced enzymatically, in situ.
Although they look very different and serve completely different functions, all cells in our body have identical DNA. However, they do not use the same genes. Certain genes are turned on and others off, depending on the type of cell and the moment in time. The "switches" are chemical changes in the building blocks of the DNA. These changes are called epigenetic modifications. One significant regulation mechanism is methylation and demethylation, meaning the attachment and removal of a methyl group (-CH3). The methylation patterns of cancer cells, for example, differ from healthy cells. During a methylation, enzymes known as methyl transferases (MTases) transfer a methyl group from S-adenosyl-L-methionine (AdoMet) to the target molecule.
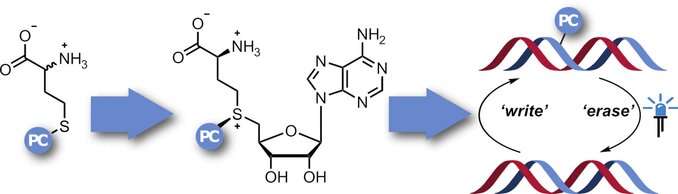
In order to study the purpose and function of this regulation more closely and determine methylation patterns, it would be useful to have "tools" to specifically inhibit methylation at targeted locations and then lift the inhibition at a defined time. To this end, a team led by Andrea Rentmeister chose to use a method known as photocaging. In this method, a "photocage" is a molecule that falls apart upon irradiation, such as a 2-nitrobenzyl group. The cage first blocks the target location, then targeted irradiation with light acts as a 'switch' to remove the blockade.
The idea was to equip AdoMet analogs with a photocage that is then transferred to the methylation sites. However, AdoMet analogs decompose in aqueous solutions and cannot enter into cells. Therefore, the team at the University of Münster wanted to produce them in situ. In the body, AdoMet is produced from the amino acid methionine through the action of the enzyme, methionine adenosyl transferase (MAT). Synthesis of the AdoMet analogs requires methionine with an attached nitrobenzyl photocage and a MAT that can use such an altered substrate. Starting with a MAT enzyme from a single-celled organism (Cryptosporidium hominis), the researchers were able to carefully change specific amino acids in the enzyme to increase the size of its hydrophobic binding cavity so that it could contain the nitrobenzyl group. A crystal structure analysis showed that the ADoMet analog is bound in the cavity of this photocaging MAT (PC-MAT). Based on this information, the team also produced a second PC-MAT based on a thermostable MAT enzyme from the archaeon Methanocaldococcus jannaschii.
Both of these PC-MATs are compatible with DNA and RNA MTases and made it possible to attach photocages to all natural methylation sites of a plasmid DNA. Irradiation with light removed the blockade.
More information: Freideriki Michailidou et al, Engineered SAM Synthetases for Enzymatic Generation of AdoMet Analogs with Photocaging Groups and Reversible DNA Modification in Cascade Reactions, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202012623
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley