Watch how cells squeeze through channels
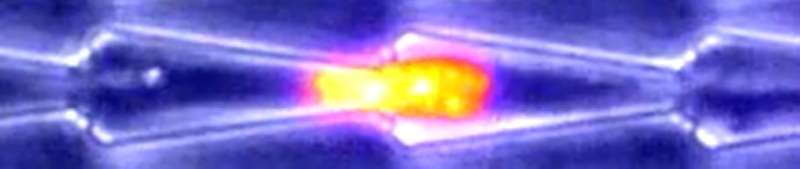
Observations of cells moving through small channels shed new light on cell migration in 3-D environments, researchers report October 6 in Biophysical Journal. The findings also reveal how cancer cells may penetrate tissues and spread throughout the body.
"Our results describe how cells can migrate and deform through confined spaces, providing potentially new ways to envision cell motility in small blood capillaries in vivo," says senior study author Daniel Riveline of the University of Strasbourg in France.
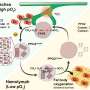
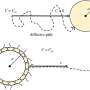
Cell migration plays a key role in a variety of biological phenomena, ranging from early development to disease processes. But cell motility has mainly been studied on flat surfaces rather than in 3-D environments similar to blood vessels and other structures commonly found in the body. To address this gap, Riveline and his collaborators studied cell motion in microfabricated channels that had either open or closed configurations (i.e., confined by three or four walls, respectively). In addition, some channels were straight, whereas others had various bottlenecks to mimic cell blockage in small veins.
As expected, fibroblasts moved freely in straight channels. But in the presence of bottlenecks, the nucleus sometimes prevented cell passage, causing pauses in cell motion. Other times, the cells anchored and pulled locally to deform the nucleus and allow cell passage. Additional results suggested that cells would not be able to change their direction of motion when entering a sufficiently small capillary, and that chemical gradients can induce directional motion.
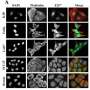
The researchers also studied the movements of oral squamous epithelial cells, including some with mutant keratin protein implicated in squamous cancers. In normal cells, keratin accumulated at the rear of the nucleus during passage through bottlenecks, potentially to facilitate deformation of the organelle. By contrast, the mutant cells could not pass through bottlenecks, indicating that defects in keratin impair motion in confined spaces, possibly by preventing the nucleus from deforming. The findings also suggest that squamous cancer cells could be blocked within small capillaries, potentially allowing them to penetrate tissues.
"Because initial arrest in the capillary is critical for tumor cells to metastasize to secondary sites in distant organs, blockage by mutant keratin may provide advantages for tumor seeding, survival, and proliferation," Riveline says. "Future studies could take this channel strategy to identify signaling networks that are modified in the context of cancer."
More information: Biophysical Journal, Le Maout and Lo Vecchio et al.: "Ratchetaxis in Channels: Entry Point and Local Asymmetry Set Cell Directions in Confinement" www.cell.com/biophysj/fulltext … 0006-3495(20)30678-0 , DOI: 10.1016/j.bpj.2020.08.028
Journal information: Biophysical Journal
Provided by Cell Press