May 1, 2020 report
Three-dimensional molecular structure of TASK channel described
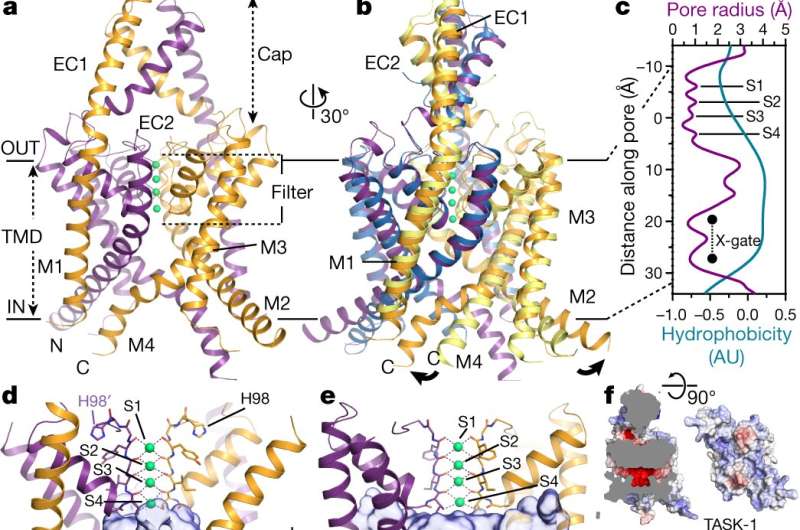
A team of researchers from the University of Oxford, the University of Marburg and Bayer Pharmaceuticals has developed a way to describe the three-dimensional molecular structure of TASK channels. In their paper published in the journal Nature, the group describe the technique they used to describe the potassium channel in detail for the first time and what they discovered as they were doing so.
TWIK-related acid-sensitive potassium (TASK) channels are members of the general potassium channel family—what sets them apart are their two-pore structure and their location—they are found in neurons, smooth vascular muscle cells and cardiomyocytes (heart muscle cells). Potassium channels are portals through cell membranes that allow or bar the transport of ions. They do so through the use of gates that physically open or close. Such channels are found in almost all living organisms—they help regulate electrical signals and nerve activity. In this new effort, the researchers sought to learn more about the structure of TASK channels, most specifically, why they are able to bind inhibitors (chemicals that urge the channels to bar the passage of ions) so well. Medical scientists have developed such inhibitors to treat some forms of respiratory failure, cardiac fibrillation and some sleep disorders such as apnea.
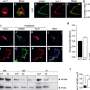
To learn more about the structure of TASK channels, the researchers studied them using X-ray crystallography—it allowed them to get an atom-to-atom view of the ion portals—and to find something unexpected. In most potassium channels, there is a gate with four helices in parallel located below a selectivity filter, but to date, no such lower gates had been seen in TASK channels. In this new effort, the researchers found evidence of just such a lower gate, which they named: X-gate. And it was created by interactions between two transmembrane helices at the entrance to the vestibule.
Study of the X-gate showed it kept inhibitors in the entrance part of the channel and that explained why inhibitors have been so notoriously difficult to wash out. They note that the discovery of the X-gate's existence also helps to explain other aspects of TASK channels and will likely help with the development of new drugs to treat lung and sleep disorders.
More information: Karin E. J. Rödström et al. A lower X-gate in TASK channels traps inhibitors within the vestibule, Nature (2020). DOI: 10.1038/s41586-020-2250-8
Journal information: Nature
© 2020 Science X Network