Team makes breakthrough in separation science with sub-Angstrom precision
An international research team that includes Vanderbilt engineers is the first to successfully separate two ions with very, very small size differences, a major advancement in separation science with widespread potential application.
The process is first to achieve solute-solute separation with sub-Angstrom precision. An Angstrom is one hundred-millionth of a centimeter, or one-tenth of a nanometer. For a sense of scale, the difference between a single Angstrom and one meter is roughly the equivalent in the difference between the width of a credit card and the radius of the Earth.
The work results from an extensive international collaboration between Vanderbilt, the Suzhou Institute of Nano-Tech and Nano-Bionics of the Chinese Academy of Sciences, Yale University and several other institutions. The advancement is reported online today in Nature Communications.
The paper's first author, Yuanzhe Liang ,is a Ph.D. student in the School of Engineering's Interdisciplinary Material Science program. Shihong Lin, assistant professor of civil and environmental engineering, is Liang's adviser and one of three corresponding authors the project.
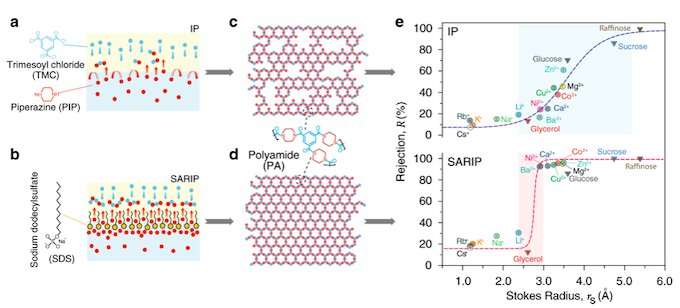
What also makes the work significant is its use of nanofabrication for solute-solute separation. Nanofiltration is very efficient, relatively mature, and has been widely used in practice. But in most cases, it is used to separate ions and small molecules from the solvent, not each other.
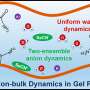
The key to achieving solute-solute separation, the authors discovered, is to use membranes with highly uniform pore size so they reject solutes larger than the pores but not just slightly smaller. But getting there isn't trivial.
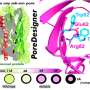
Current state-of-art commercial nanofiltration membranes are fabricated using interfacial polymerization, in which two chemical precursors, one in the water phase and the other in the oil phase, react. The reaction creates a thin film of polymer at the water/oil interface that acts as the active separation layer. This layer has Angstrom-scale pores, but the complex process happens within seconds and makes obtaining smaller, uniform pores very challenging.
The team's novel method uses a dynamic, self-assembled network of surfactants to facilitate faster and more homogeneous diffusion of specific molecules, or monomers, across the water/oil interface, when the monomers bond with each other to form a polymer. The key to "surfactant assembly regulated interfacial polymerization," or SARIP, as it is called, is in adding the right kinds of surfactants to promote the formation of a highly organized network of very narrow and highly uniform pore size at the water/oil interface.
The team evaluated which types of surfactants work the best and demonstrated that the approach also works with other pairs of surfactants, or precursors.
Nanofiltration, which is more efficient and uses less energy than other technologies, such as electrochemical and thermal separations, already is in wide use, creating vast opportunities across many sectors for the team's discovery.
"Precise separation of ions and small molecules using membranes will have transformative impacts on energy, water, chemical, and pharmaceutical industries," the authors said.
More information: Yuanzhe Liang et al. Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1 Å precision separation, Nature Communications (2020). DOI: 10.1038/s41467-020-15771-2
Journal information: Nature Communications
Provided by Vanderbilt University