May 11, 2020 feature
Cation-induced shape programming and morphing in protein-based hydrogels
![Schematics of the fixation process. (Left) BSA-based protein hydrogels are fabricated using a light-activated reaction, in the presence of ammonium persulfate (APS) and tris(bipyridine) ruthenium(II) chloride [Ru(bpy)3]2+. (Right) Following synthesis, the protein hydrogels are exposed to Zn2+ or Cu2+, which reversibly increases their stiffness by up to 17-fold. This stiffening effect can be used for shape programming. Credit: Science Advances, doi: 10.1126/sciadv.aba6112 Cation-induced shape programming and morphing in protein-based hydrogels](https://scx1.b-cdn.net/csz/news/800a/2020/cationinduce.jpg)
Smart materials or advanced materials that can memorize a temporary shape and morph in response to a stimulus can revolutionize medicine and robotics. In a new study now on Science Advances, Luai R. Khoury and a research team in the department of physics at the University of Wisconsin-Miluwaukee U.S. introduced an innovative approach to program protein hydrogels and induce shape changes at room temperature in aqueous solutions. The team demonstrated their approach using hydrogels made of serum albumin, the most abundant protein in blood plasma. The scientists synthesized the protein in a cylindrical or flower shape and programmed the gels into a spring or ring shape. They performed the programming by changing the stiffness of the material by inducing the adsorption of Zinc (Zn2+) or Copper (Cu2+) cations. The programmed biomaterials could morph back into their original shape as the cations diffused outside of the hydrogel material. The method is an innovative strategy to program protein-based hydrogels to potentially act as robotic actuators.
Dynamic biomaterials with conformational changes can facilitate artificial tissue structures for morphological transformation and soft robotics in order to react and change in response to their environment. The most common shape-morphing materials are based on polymers that require switching between a stiff and soft phase. Such materials generally rely on two or more network skeletons that share the same 3-dimensional (3-D) space or maintain a chemical response to small ions. Programming is defined as the ability to fix a temporary shape in a material and the process requires a reversible increase in stiffness. The initial shape recovery can switch from a stiff to a soft phase, typically realized by changing the temperature, pH or photo-switching to compromise the integrity of the secondary network.
Khoury et al. previously introduced a method to form shape-memory in protein-based hydrogels, where proteins formed the primary network in a water-rich environment, by stiffening the hydrogels with adsorbed polyelectrolytes. In this approach, the team produced protein hydrogels using bovine serum albumin, which is homologous to human serum albumin—the most abundant blood plasma protein. They programmed the hydrogel by stiffening induced via a secondary network made of positively charged polyelectrolytes, and stimulated a shape change by initiating the unfolding response of the protein domains in chemical denaturants.
The strategy allowed complete recovery on removing the denaturant and this transition was highly repeatable, but polyelectrolyte adsorption was irreversible and resulted in a change in stiffness. In this work, the scientists used divalent cations to stiffen protein-based hydrogels and programmed them into a variety of shapes that successfully morphed back to their original shape via simple diffusion. The research team explored the mechanical change to induce increased stiffness and program protein-based biomaterials into a variety of shapes. The newly programmed protein-based hydrogels with small ions formed an important step to engineer biocompatible biomaterials with adjustable structures.
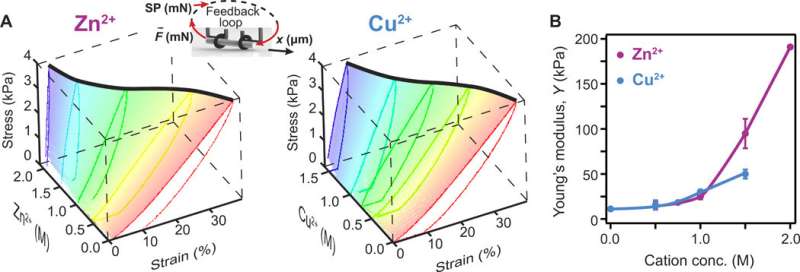
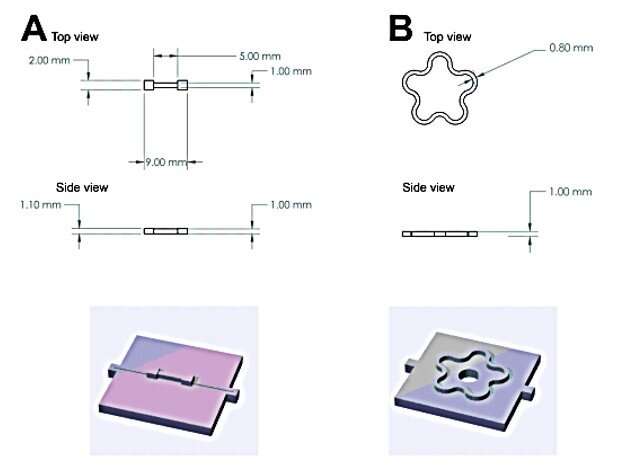
A series of reactions can yield protein-based hydrogels including cross-linking strategies based on treatments with glutaraldehyde, enzymatic reactions, or photoactivation. Khouri et al. used photoactivation to form the protein-based hydrogels made of BSA, the reaction produced covalent carbon-carbon bonds. They tested a range of concentrations for positively charged ions to increase the stiffness of protein hydrogels for shape programming, and measured the change in stiffness using a force-clamp rheometric apparatus. The team chose 2 mM as the starting concentration to produce complete cross-linking for BSA and the resulting hydrogels showed reversible behavior without plastic deformation. The BSA hydrogels showed an increased stiffness of up to 5-fold when treated with Cu2+ and a 17-fold stiffness in the presence of Zn2+; several orders of magnitude greater than those reported for gels treated with polyelectrolytes, allowing for more complex programmed shapes. The stiffening effect relied on the solution concentration, where Zn2+ was more soluble in water and therefore more advantageous than Cu2+. The BSA-based hydrogels had an increased toughness and failure stress due to increased cation concentrations. The toughness represented the ability of the material to adsorb energy and deform without fracture. However, irreversible breaking of covalent bonds was a limiting factor for extensions and therefore the hydrogel required further refinement.
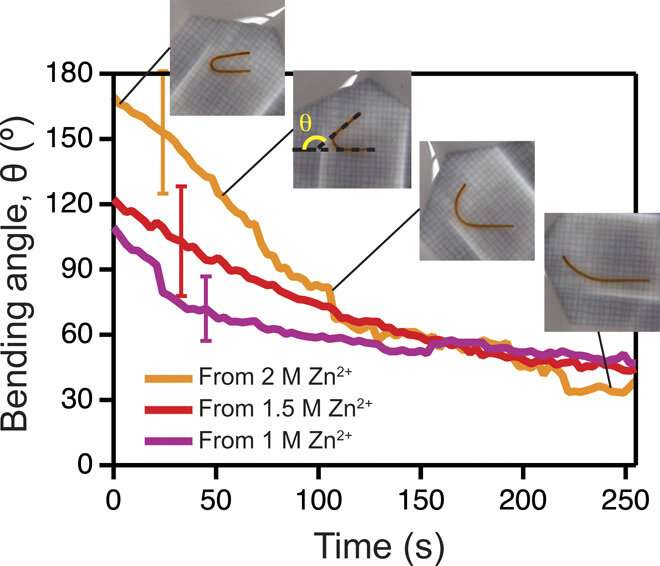
Since the dynamics of morphing from the programmed shape to the initial shape directly depended on cation diffusion outside the biomaterial, Khoury et al. monitored the phenomenon using a cylindrical U-shaped hydrogel. The shape of hydrogel depended on the amount of stiffening induced by dosing with cations. The scientists obtained a temporary shape by combining ionic cross-linking and stable divalent cations in the material. They then programmed cylindrically casted biomaterials into a spring shape and flower casted materials into a ring shape. Cations in the medium induced strong enough stiffening followed by morphing from a ring to a flower shape.
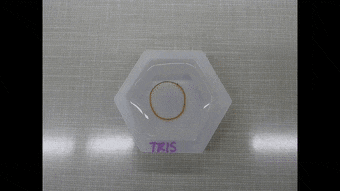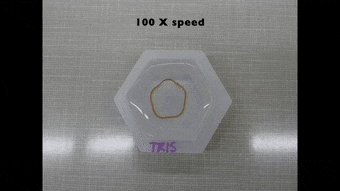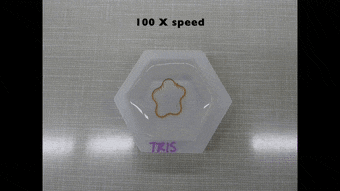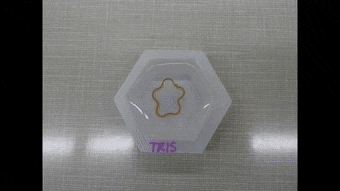
Polymer-based hydrogels alone have a variety of applications in shape memory and shape morphing applications, although they are not as structurally diverse as naturally occurring proteins. In this new approach, Luai R. Khoury and colleagues developed protein-based hydrogels to facilitate the best of both worlds. The approach relied on Zn2+ and Cu2+ cations to induce stiffening so as to program a permanent shape into a new temporary configuration. They have also made the protocols of the study broadly accessible via Bio-protocol. Diffusion of the ions outside the material allowed the team to recover the original structure. They aim to use Zn2+ predominantly in future work due to higher biocompatibility compared to Cu2+. The approach preserved the functionality of proteins by forming the skeleton of the hydrogel and remarkably combined biodiversity with reversible programming capability.
More information: Luai R. Khoury et al. Cation-induced shape programming and morphing in protein-based hydrogels, Science Advances (2020). DOI: 10.1126/sciadv.aba6112
Jeong-Yun Sun et al. Highly stretchable and tough hydrogels, Nature (2012). DOI: 10.1038/nature11409
Haoran Fu et al. Morphable 3-D mesostructures and microelectronic devices by multistable buckling mechanics, Nature Materials (2018). DOI: 10.1038/s41563-017-0011-3
Journal information: Science Advances , Nature , Nature Materials
© 2020 Science X Network