April 28, 2020 feature
Porous carbon nanofibers demonstrate exceptional capacitive deionization
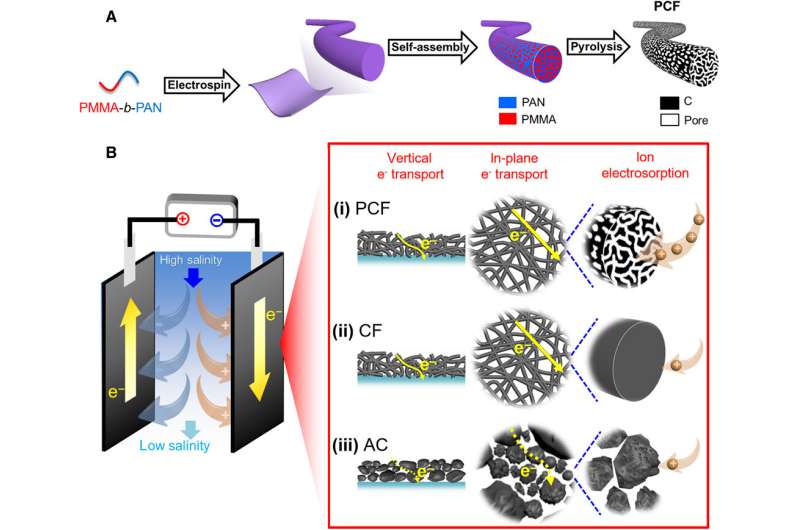
Capacitive deionization (CDI) is energetically favorable to deionize water, but existing methods are limited by their desalination capacities and time-consuming cycles due to insufficient ion-accessible surfaces and slow electron/ion transport. In a new report on Science Advances, Tianyu Liu and a research team in the departments of chemistry, civil and environmental engineering, and nanoscience at Virginia Tech, U.S., demonstrated porous carbon fibers (PCF) as an effective CDI material. They derived the PCFs from microphase-separated poly(methyl methacrylate)-block-polyacrylonitrile (PMMA-b-PAN). The resulting PCFs maintained abundant and uniform mesopores interconnected with micropores to form a hierarchical porous structure with a large, ion-accessible surface area and high desalination capacity. The continuous carbon fibers and interconnected porous network allowed fast electron/ion transport to maintain a high desalination rate. The work highlights the promise of copolymer-based PCF for high capacity and high rate CDI.
The increasing withdrawal and uneven distribution of freshwater impose critical challenges to technical and socio-economic development. Desalination is a promising approach based on a vast reservoir of seawater to address the freshwater shortage. Reverse osmosis and thermal distillation are widely practiced techniques to process seawater or brackish water of high salt concentrations, although such methods are energy intensive and costly when salt concentrations are low. As an alternative, capacitive deionization (CDI) can remove ions through electrosorption or pseudocapacitive reactions to desalinate water of low salt concentrations.
Materials scientists use porous carbons as primary CDI electrode materials due to their high electrical conductivity, large surface area, tailorable structure and excellent stability. Examples include activated carbon (AC), graphene aerogels and macroporous carbons derived from biomass. However, the desalination capacities and rates of such materials remain to be improved. Based on the limited performance of microporous and macroporous materials, Liu et al. hypothesize that carbon fibers will be able to achieve high rates of desalination capacity due to the interconnected hierarchical architecture. In this work, the team demonstrated porous carbon fibers (PCFs) as superior electrode materials for capacitive deionization. The innovation of the technique here relied on the design of the carbon electrode precursor at the molecular level. Liu et al. used a block copolymer to create PCFs through electrospinning, oxidation, stabilization, and pyrolysis. The resulting large effective desalination surface area with abundant and uniform architecture enhanced desalination capacity by allowing rapid electron transport and fast ion diffusion.
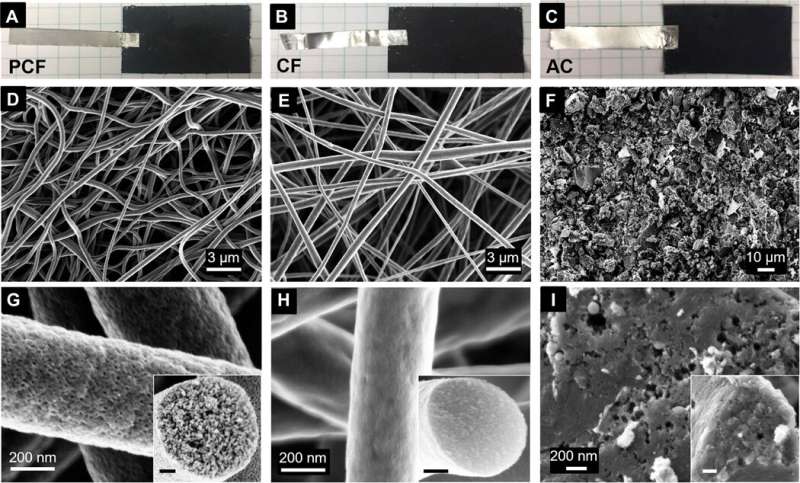
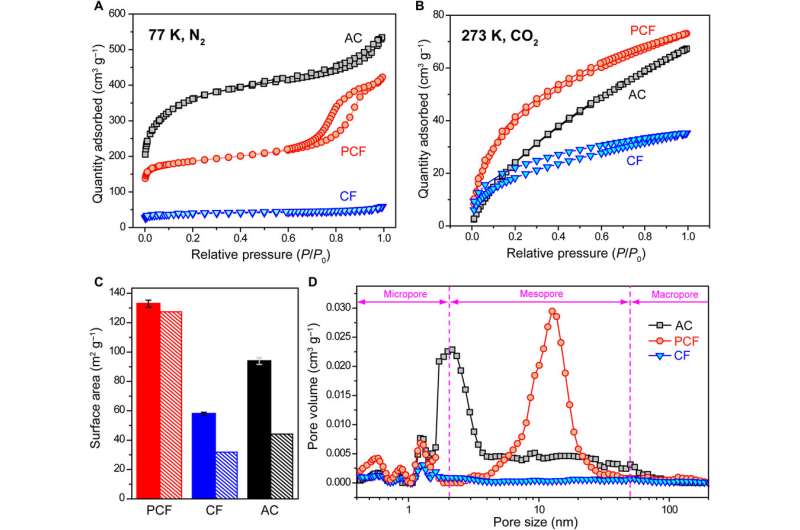
To design materials for CDI, the team studied three carbon materials, including block copolymer-based PCF (porous carbon fibers) with large ion-accessible surface area. The team also tested industrial PAN (polyacrylonitrile)-based carbon fibers (CF) and activated carbon (AC). The fibrous carbon and interconnected mesopores allowed continuous and effective transport pathways for electrons and ions, while reducing the internal resistance of desalination in cells and improving the rate of desalination. In contrast, the other materials had limited surface area for electrosorption of ions and a deteriorated desalination rate. The team then adhered all three materials; PCF, CF and AC to tin-plated copper tapes and used them as electrodes in CDI cells. Using scanning electron microscopy (SEM) images they noted distinct appearances for the three diverse materials. Based on the initial results they expected PCF to exhibit the highest rate of desalination.
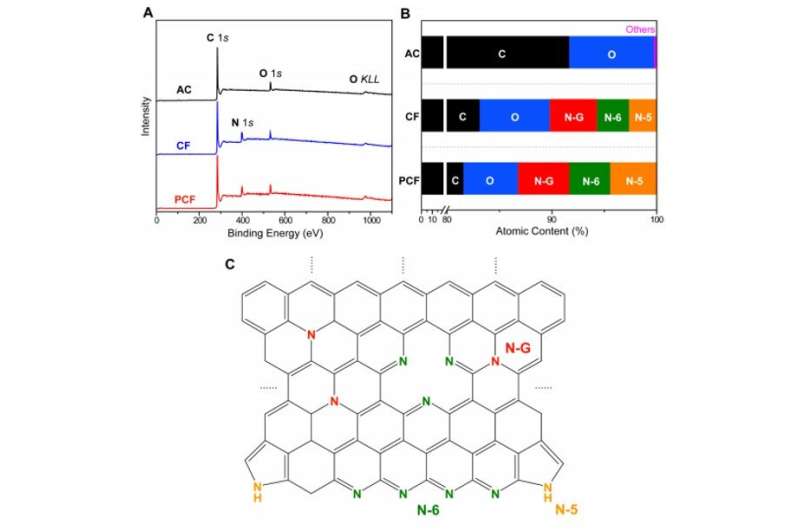
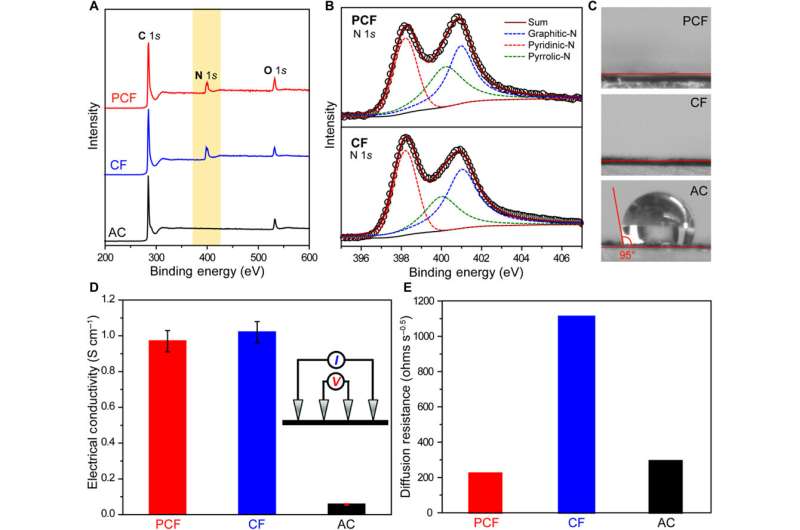
The scientists next conducted a series of experiments to understand the chemical and electrical properties of all three materials. After measuring the water contact angle between the surface, they noted a large contact angle for AC substrates, which represented a hydrophobic (water-repelling) interface—undesirable to desalinate aqueous solutions. Meanwhile, without any conductive additives, the PCF and CF materials were highly electrically conductive according to electrochemical impedance spectroscopy and four-point probe measurements. Based on multiple characteristics including hierarchical porous structures, effective surface area, high electrical conductivity and low diffusion resistance, the team decided PCF would be an excellent electrode material for CDI.
They demonstrated the deionization capability of PCF by desalinating two sources of water including artificial brackish water with sodium chloride (NaCl) and synthetic tap water with NaCl in conical cells, with two symmetric electrodes. They determined the concentrations using ion chromatography and noted the NaCl concentration of tap water to have dropped to an ultra-low concentration after five deionization cycles. Liu et al. further quantified the desalination capacity and rate of PCF using single-cycle deionization at an applied bias voltage of 1.0 V across the two PCF electrodes to observe decreasing salt concentrations from 501.2 to 477.5 mg/L. In comparison, CDI cells containing CF and AC only showed a slight decrease in salt concentration at the same voltage bias. The desalination capacity of PCF, at 30 mgNaCl gPCF−1, outperformed other carbon CDI electrodes and reached a maximum desalination rate of 38 mg g−1 min−1 approximately 40 times faster than carbon nanotubes, graphene, CFs and other three-dimensional porous carbons.
The energy consumption accompanying PCF was also low, and the versatile material could remove other common cations in water, including potassium ions (K+), magnesium ions (Mg2+) and calcium ions (Ca2+). Chemical reactions did not alter the surface of PCF due to the electrical double-layer of CDI cells, allowing the surface to retain its desalination capacity without signs of degradation or substantial loss after repeated charge-discharge cycles. In this way, Tianyu Liu and colleagues highlighted block copolymer-based PCF as a high performance electrode material for CDI, while maintaining an ultrahigh desalination capacity, surpassing other state-of-the-art carbon materials. Liu et al. credited the ultrafast rate and high capacity of desalination to the combined structural, physical and electrical properties of PCF. In the future, Liu et al. will investigate how the properties of PCF influence the desalination performance—they expect a positive correlation between surface properties of the material and capacitive deionization. The researchers propose additional engineering strategies to design efficient flow through continuous desalination cells using PCF to further boost the capacity and rate of desalination.
More information: Tianyu Liu et al. Exceptional capacitive deionization rate and capacity by block copolymer–based porous carbon fibers, Science Advances (2020). DOI: 10.1126/sciadv.aaz0906
M. Rodell et al. Emerging trends in global freshwater availability, Nature (2018). DOI: 10.1038/s41586-018-0123-1
Zhi Yi Leong et al. Three-dimensional graphene oxide and polyvinyl alcohol composites as structured activated carbons for capacitive desalination, Desalination (2017). DOI: 10.1016/j.desal.2017.07.018
Journal information: Science Advances , Nature
© 2020 Science X Network