April 24, 2020 feature
Highly sensitive nanosensor detects subtle potassium changes in the brain
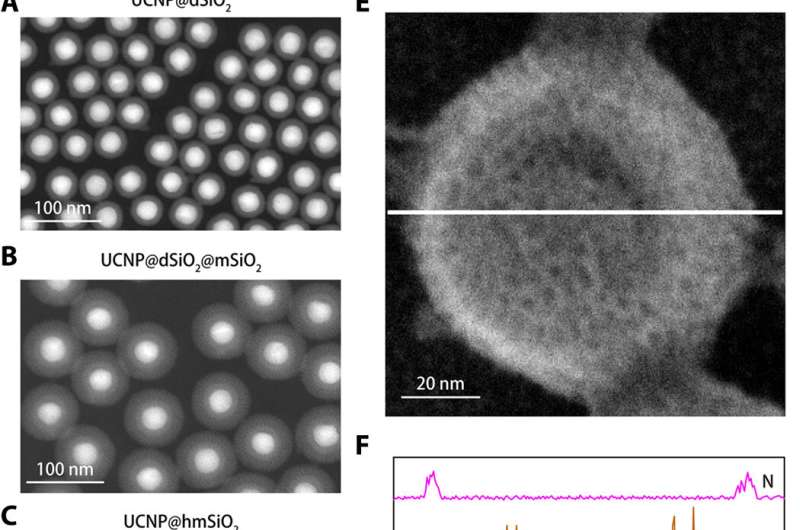
![Design and sensing mechanism of the K+ nanosensor. (A) Schematic illustration for the synthesis of the nanosensor. The NaYF4:Yb/Tm@NaYF4:Yb/Nd (UCNP) core was synthesized and coated with a dense silica layer and a successive mesoporous silica shell. Etching away of the dense silica layer forms a hollow cavity that allows the loading of PBFI. The nanosensor was lastly coated with the K+-selective filter membrane. (B) Schematics showing a magnified view of the nanosensor [from the red dotted box in (A)] and its K+ sensing mechanism. The filter membrane layer allows only K+ to diffuse into and out of the nanosensor, thus excluding the interference from other cations. Once diffused into the nanosensor, K+ will bind to PBFI immediately. Upon NIR irradiation, the upconverted UV light from the UCNPs excites PBFI, leading to the emission of K+-bonded PBFI. Credit: Science Advances, doi: 10.1126/sciadv.aax9757 Highly sensitive nanosensor detects subtle potassium changes in the brain](https://scx1.b-cdn.net/csz/news/800a/2020/2-highlysensit.jpg)
Researchers have developed a number of potassium ion (K+) probes to detect fluctuating K+ concentrations during a variety of biological processes. However, such probes are not sensitive enough to detect physiological fluctuations in living animals and it is not easy to monitor deep tissues with short-wavelength excitations that are in use so far. In a new report, Jianan Liu and a team of researchers in neuroscience, chemistry, and molecular engineering in China, describe a highly sensitive and selective nanosensor for near infrared (NIR) K+ ion imaging in living cells and animals. The team constructed the nanosensor by encapsulating upconversion nanoparticles (UCNPs) and a commercial potassium ion indicator in the hollow cavity of mesoporous silica nanoparticles and coated them with a K+ selective filter membrane. The membrane adsorbed K+ from the medium and filtered away any interfering cations. In its mechanism of action, UCNPs converted NIR to ultraviolet (UV) light to excite the potassium ion indicator and detect fluctuating potassium ion concentrations in cultured cells and in animal models of disease including mice and zebrafish larvae. The results are now published on Science Advances.
The most abundant intracellular cation potassium (K+) is extremely crucial in a variety of biological processes including neural transmission, heartbeat, muscle contraction and kidney function. Variations in the intracellular or extracellular K+ concentration (referred herein as [K+]) suggest abnormal physiological functions including heart dysfunction, cancer, and diabetes. As a result, researchers are keen to develop effective strategies to monitor the dynamics of [K+] fluctuations, specifically with direct optical imaging.
Most existing probes are not sensitive to K+ detection under physiological conditions and cannot differentiate fluctuations between [K+] and the accompanying sodium ion ([Na+]) during transmembrane transport in the Na+/K+ pumps. While fluorescence lifetime imaging can distinguish K+ and Na+ in water solution, the method requires specialized instruments. Most K+ sensors are also activated with short wavelength light including ultraviolet (UV) or visible light—leading to significant scattering and limited penetration depth when examining living tissues. In contrast, the proposed near-infrared (NIR) imaging technique will offer unique advantages during deep tissue imaging as a plausible alternative.
Designing the K+ nanosensor and characterizing its structure
To engineer the nanosensor, Liu et al. encapsulated upconversion nanoparticles (UCNPs) and a commercial K+ indicator—potassium-binding benzofuran isophthalate (PBFI) into the core of mesoporous silica nanoparticles (MSNs). The UCNPs were able to convert NIR light into UV light and excite the acceptor of the K+ indicator through luminescence resonance energy transfer. They shielded the outer surface of silica nanoparticles with a thin layer of K+ selective filter membrane with micropores created from carbonyl oxygen for specificity. The setup favored the free transfer of K+ through the membrane pore, while preventing other biologically relevant cations from diffusing through. The technique allowed them to detect slight fluctuations in [K+] in the solution. The team used transmission electron microscopy (TEM) to observe the well-controlled structure and appearance of the nanoparticles during each step of nanosensor construction. Dynamic light scattering confirmed the presence of a filter membrane on the surface of the shielded nanosensor.
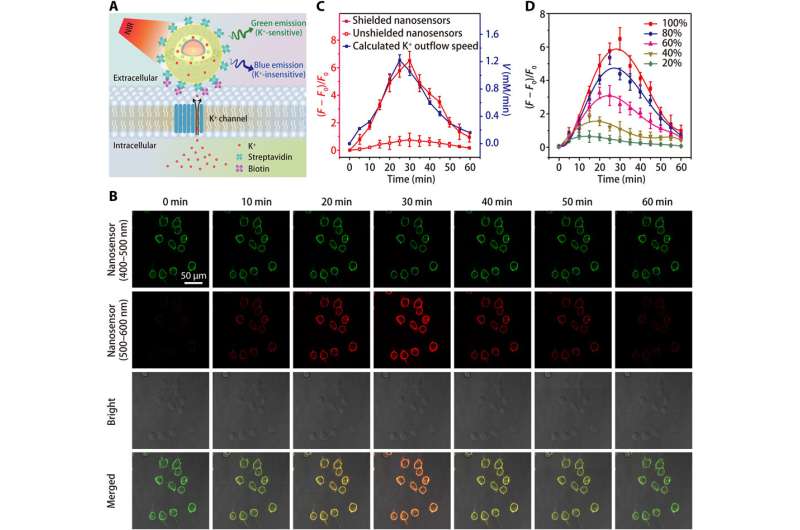
Performance of the nanosensor in water and during [K+] fluctuations in cells.
The team tested the enhanced sensitivity of the shielded nanosensor in a physiological range (0 to 150 mM) and showed a 12-fold increase in fluorescence intensity compared to unshielded nanosensors. The K+ probes had to display high selectivity against Na+, which Liu et al. verified using the shielded nanosensor by rapidly detecting consistent fluorescence sensitivity to fluctuating [K+], while remaining unaffected by increasing [Na+].
Since living cells rely on the sodium-potassium adenosine triphosphatase (Na+/K+ pump) to maintain a steep [K+] gradient across their plasma membrane, the process is partially responsible for the cell's energy expenditure. Defects in cellular energy metabolism can lead to a loss of the [K+] gradient, while giving rise to extracellular [K+] known as [K+]0, which the scientists monitored to obtain a valuable indicator of cell viability and growth. Thereafter, they increased the specificity of the nanosensor to detect cell death or proliferation rates by grafting polyethylene glycol (PEG) on the surface of nanosensors in a culture medium containing the human embryonic kidney 293 cell line. They then optimized the protocol by anchoring large numbers of nanosensors onto cell membranes using streptavidin-conjugated nanosensors to biotin-modified cells. The results highlighted improved sensitivity of shielded nanosensors to continuously monitor the K+ efflux.

Spreading waves in the mouse intact brain
The team then applied the shielded nanosensor to investigate cortical spreading depression (CSD) in the mouse brain as a wave-like propagation of neural activity. The process typically involves a slow propagation release of K+ in the cortical surface and could be triggered in the mouse brain via potassium chloride (KCl) incubation. The scientists simultaneously monitored the local field potential and optical signal through the surgical cranial window and observed a wave of increasing [K+]0 propagate gradually across the cortex after stimulation. Liu et al. did not observe a wave in mice injected with unshielded nanosensors, indicating the importance of the outer filter for improved sensitivity of the nanosensor. The recorded wave velocity did not vary significantly from the values obtained using blood oxygen-level dependent magnetic resonance imaging (MRI) in patients with migraine aura.
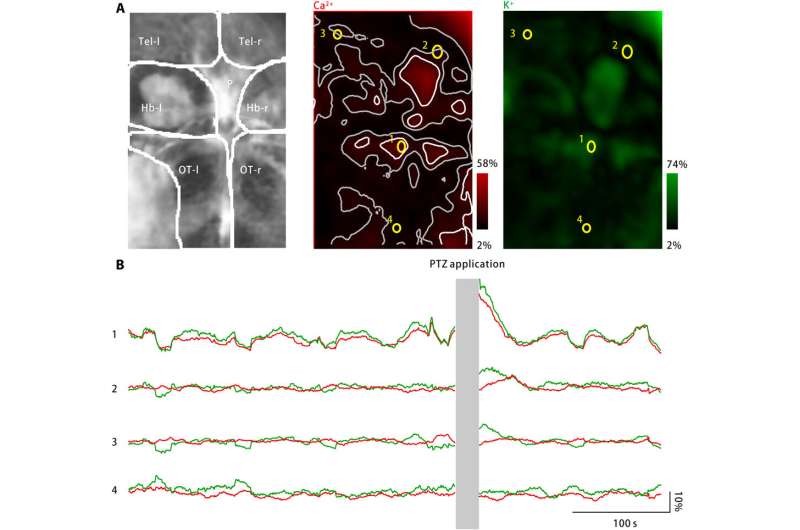
To extend applications of the nanosensor, Liu et al. monitored neuronal calcium levels and extracellular potassium concentrations using zebrafish larvae. While a large increase in the extracellular potassium concentration can cause intense neuronal activation to cause CSD and epilepsy, no direct evidence exists to show changes in extracellular potassium during the disease. The team therefore engineered a disease model using zebrafish larvae to increase extracellular potassium concentrations and observed disease characteristic neuronal activation in specific brain regions.
In this way, Jianan Liu and colleagues engineered a potassium ion nanosensor with extremely high sensitivity and selectivity. The external coating of a selective filter membrane enhanced the selectivity, sensitivity, and kinetics of the device for rapid and quantitative [K+] detection in living cells and intact brains. The shielded nanosensor will have broad applications in brain research to improve the understanding of abnormal [K+]-related diseases. The method alongside optical fiber-based endoscope and photometry will allow real-time potassium imaging in freely moving animals.
More information: Jianan Liu et al. A highly sensitive and selective nanosensor for near-infrared potassium imaging, Science Advances (2020). DOI: 10.1126/sciadv.aax9757
Prashant Padmawar et al. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator, Nature Methods (2005). DOI: 10.1038/nmeth801
Michelle L. Gumz et al. An Integrated View of Potassium Homeostasis, New England Journal of Medicine (2015). DOI: 10.1056/NEJMra1313341
Journal information: Science Advances , Nature Methods , New England Journal of Medicine
© 2020 Science X Network