Lead-halide perovskites with enhanced luminescence and stability
Lead halide perovskites are materials that emit light with a range of colors, but also suffer from poor moisture stability. Scientists in China demonstrated a new synthetic technique that can enable perovskites to emit bright fluorescent light by adding a dose of water, even when submerged under water for more than a year. Successful fabrication of the hydrated crystals as phosphors within light-emitting devices indicates their potential for industrial purposes.
In recent years, lead halide perovskites have emerged as promising materials for photovoltaics and light-emitting diodes (LEDs) due to their attractive optical and electrical properties, such as high photoluminescence (PL) quantum yield (QY), narrow emission spectrum, tuneable emission wavelength, high absorption coefficient, and long carrier diffusion length. Profound developments have been witnessed in the fields of solar cells, solid-state light-emitting diodes, photodetectors, and lasers. However, the poor stability of LHPs, especially in water and polar solvents, remains a crucial issue that hampers their applications.
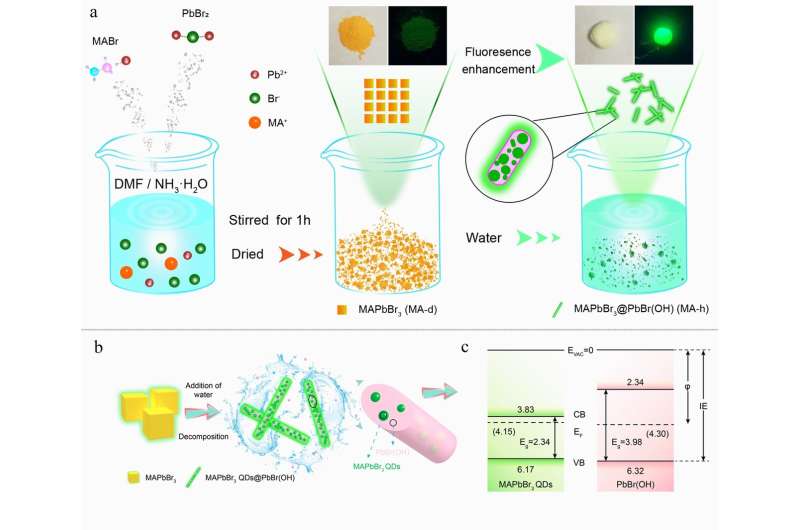
In a new paper published in Light Science & Applications, scientists from Zhengzhou University, China, and co-workers developed a new synthetic method by which the PL QY of perovskites can be increased from 2.5% to 71.54% by addition a dose of water and decreases minimally in aqueous solution in one year. In addition, the as-synthesized MAPbBr3@PbBr(OH) can maintain their luminescence in many kinds of solvents and also exhibit excellent ambient, thermal and photo-stabilities. The enhanced stability and PL QY can be attributed to the water-induced MAPbBr3@PbBr(OH). PbBr(OH) passivated the defects of the MAPbBr3 QDs and confined carriers within the QDs so that MAPbBr3@PbBr(OH) could reach high emission efficiency; additionally, PbBr(OH) can prevent the exposure of the QDs to air and moisture, thus increasing stability.
"The finding that PL QY of perovskites can be increased by the addition of water is amazing, and the reason for enhanced PL QY and stability can be attributed to the formation of stable and larger bandgaps PbBr(OH) on the surface of the lead halide perovskites quantum dots after the addition of water. PbBr(OH) passivated the defects of the MAPbBr3 QDs and prevented the exposure of the QDs to air and moisture, thus increasing efficiency and stability."
"We note that this strategy is universal to methylamino lead halide perovskites (MAPbBr3), formamidine lead halide perovskites (FAPbBr3), inorganic lead halide perovskites (CsPbBr3), etc" they added.
"Since the as-prepared MAPbBr3@PbBr(OH) has high fluorescence efficiency and stability, this will stimulate research interest in the field of lasers, LED and so on. This efficient approach for the synthesis of ultrastable and highly efficient luminescent perovskites will push forward their practical applications," the scientists forecast.
More information: Kai-Kai Liu et al, Water-induced MAPbBr3@PbBr(OH) with enhanced luminescence and stability, Light: Science & Applications (2020). DOI: 10.1038/s41377-020-0283-2
Journal information: Light: Science & Applications
Provided by Chinese Academy of Sciences