A novel L-fucose metabolic pathway from strictly anaerobic and pathogenic bacteria
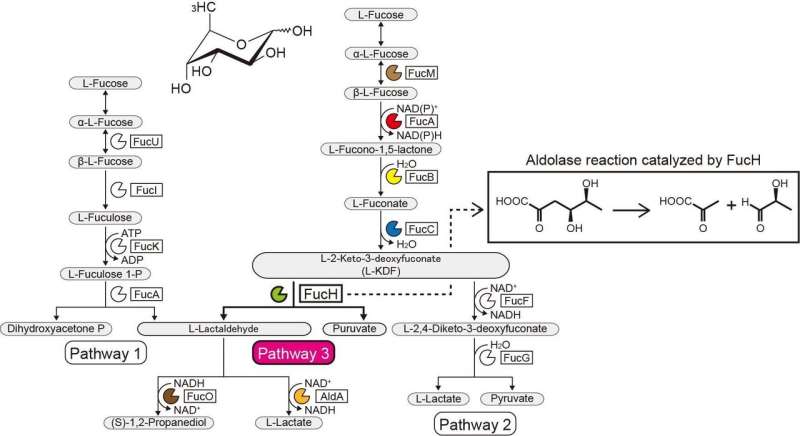
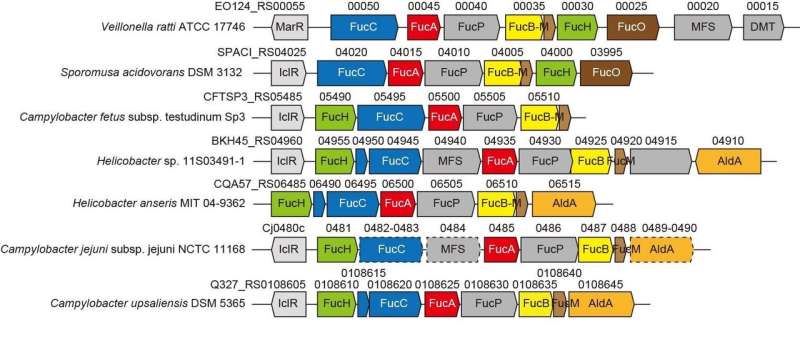
The genetic context in bacterial genomes and screening for potential substrates can help identify the biochemical functions of bacterial enzymes. The Gram-negative, strictly anaerobic bacterium Veillonella ratti possesses a gene cluster that appears to be related to L-fucose metabolism and contains a putative dihydrodipicolinate synthase DHDPS/NAL protein (FucH). Here, screening of a library of 2-keto-3-deoxysugar acids with this protein and the biochemical characterization of neighboring genes revealed that this gene cluster encodes enzymes in a previously unknown "Route I" non-phosphorylating L-fucose pathway.
Previous studies of other aldolases in the DHDPS/NAL protein superfamily used only limited numbers of compounds, and the approach reported here enabled elucidation of the substrate specificities and stereochemical selectivities of these aldolases and the comparison of them with those of FucH. According to the aldol cleavage reaction, the aldolases were specific for (R)- and (S)-stereospecific groups at the C4 position of 2-keto-3-deoxysugar acid, but had no structural specificity or preference for the methyl groups at the C5 and C6 positions, respectively.
This categorization corresponded to the (Re)- or (Si)-facial selectivity of the pyruvate enamine on the (glycer)aldehyde carbonyl in the aldol-condensation reaction. These properties are commonly determined by whether a serine or threonine residue is positioned at the equivalent position close to the active site(s), and site-directed mutagenesis markedly modified C4-OH preference and the selective formation of a diastereomer. We propose that substrate specificity of 2-keto-3-deoxysugar acid aldolases was convergently acquired during evolution and report the discovery of another L-2-keto-3-deoxyfuconate aldolase involved in the same non-phosphorylating L-fucose pathway in Campylobacter jejuni.
More information: Seiya Watanabe. Characterization of l-2-keto-3-deoxyfuconate aldolases in a nonphosphorylating l-fucose metabolism pathway in anaerobic bacteria, Journal of Biological Chemistry (2019). DOI: 10.1074/jbc.RA119.011854
Journal information: Journal of Biological Chemistry
Provided by Ehime University




















