Electrocatalyst exhibits superb water splitting activity
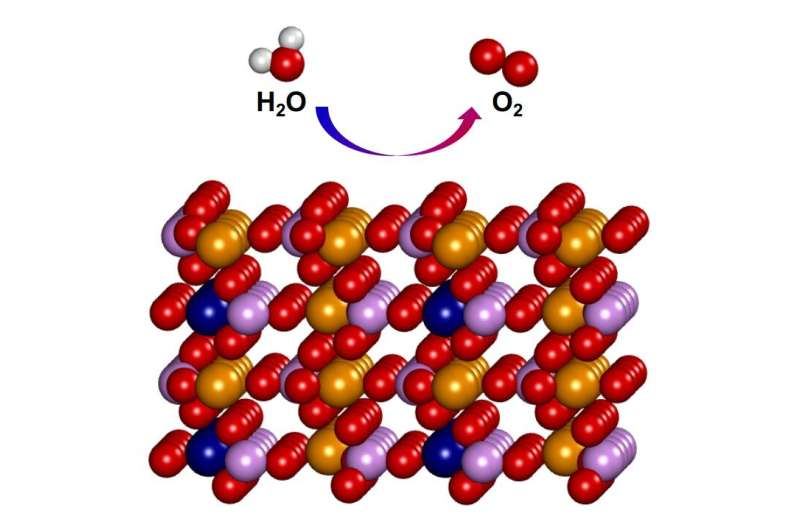
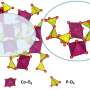
A recent study, affiliated with South Korea's Ulsan National Institute of Science and Technology (UNIST) has reported a phosphate-based electrocatalyst of Fe3Co(PO4)4/reduced-graphene-oxide (rGO) (1) for OER, which is predicted to be highly active by density functional theory (DFT).
There has been much interest in 'hydrogen that breaks water molecules apart to generate electricity' as an environmentally friendly energy to replace fossil fuels. At the same time, it is important to increase the efficiency of the water decomposition reaction to use less electricity, and inexpensive high-performance catalysts have been developed to help the important oxygen-generating reactions.
A research team, led by Distinguished Professor Kwang S. Kim in the School of Natural Sciences at UNIST has reported a phosphate-based electrocatalyst of Fe3Co(PO4)4/reduced-graphene-oxide (rGO) (1) for OER, which is predicted to be highly active by density functional theory (DFT). The new catalyst is eye-catching, with a 25% performance improvement over commercially available expensive catalysts.
In the water decomposition reaction, hydrogen and oxygen producing reactions occur simultaneously. However, the oxygen generation reaction of the two is relatively slow to lower the efficiency of the overall water decomposition reaction. In order to overcome this problem, iridium oxide (IrO₂) and ruthenium oxide (RuO₂) are used as catalysts for oxygen generation reactions to increase the reaction rate, but they are less stable than excellent performance. In addition, expensive noble metals such as iridium and ruthenium have the limitation that the main components.
The team developed a new oxygen-generating catalyst that uses inexpensive materials and is highly efficient and stable. Sultan is a material in which iron (Fe), cobalt (Co) and phosphoric acid (P) are placed on a graphene oxide support designed by a UNIST chemistry research fellow. According to the research direction, Hamilan, a UNIST chemistry researcher, used a supercomputer to calculate materials of various compositions that iron and cobalt could combine with phosphoric acid.
In case of a phosphate-based electrocatalyst, the oxygen evolution reaction takes place on iron and cobalt atoms. The distribution of electrons and chemical bonds around these atoms determine the efficiency of the oxygen evolution reaction. For the newly developed catalysts, the added phosphoric acid was calculated to optimize this fraction. The team synthesized these theoretically predicted materials and demonstrated them experimentally.
The new catalyst is more than 25% more efficient than commercial iridium oxide catalysts. Catalytic efficiency is assessed as "overvoltage," the amount of additional electrical energy that goes into the reaction. Iridium oxide required 303 millivolts (mV) when obtaining a current density of 100 milliamps (mA) per 1 cm 2 of catalyst, but only 237 mV for the new catalyst. This value is close to the theoretically predicted value.
The newly synthesized material has excellent stability as well as performance. After more than 5000 reactions did not change significantly structurally, and even after the reaction for 70 hours did not degrade the reactivity. In addition, the graphene oxide support constituting the catalyst compensated for the low electrical conductivity of iron / cobalt and phosphoric acid, which showed better reactivity.
"Through this study, we have developed a catalyst that is much more oxygen-producing than an expensive commercial catalyst and is several hundred times cheaper," says Distinguished Professor Kim. "It will be useful for developing catalysts for various eco-friendly energy materials such as fuel cells."
The findings of this research have been published in the November 2019 issue of Nature Communications.
More information: Siraj Sultan et al. Superb water splitting activity of the electrocatalyst Fe3Co(PO4)4 designed with computation aid, Nature Communications (2019). DOI: 10.1038/s41467-019-13050-3
Journal information: Nature Communications