Chemist synthesizes new compounds with strong antidiabetic properties
A RUDN University chemist has synthesized new derivatives of 1,2,4-triazole that exhibit antidiabetic properties. Experiments showed that these compounds work better than acarbose, a widely used hypoglycemic drug, and demonstrate antioxidant properties. In the future, they can be used to develop drugs against type 2 diabetes. The article is published in the journal Bioorganic Chemistry.
In the small bowel, the polymeric structure of starch splits into simpler oligosaccharides thanks to an enzyme called α-amylase. Further, under the action of the enzyme α-glucosidase, the obtained oligosaccharides transformed into glucose and other monosaccharides. If you inhibit one or both of these enzymes, the rate of absorption of carbohydrates will decrease, and consequently, the concentration of glucose in the blood will decrease. This antidiabetic effect led the researchers to pay more attention to the search and synthesis of α-amylase and α-glucosidase inhibitors.
Youness El Bakri from RUDN University and colleagues synthesized 17 new derivatives of 1,2,4-triazole. They belong to the class of heterocycles, organic compounds that contain "rings" of carbon atoms and atoms of other elements. Due to their structure, which resembles that of natural products, heterocyclic compounds often represent interesting biologically active properties.
The RUDN University chemists established the structure of new heterocyclic compounds using X-ray diffraction analysis and spectral methods. Then they studied the inhibitory activity of all 1,2,4-triazole derivatives in vitro and compared them to acarbose, a hypoglycemic drug that inhibits α-glucosidase. All new compounds proved to be potent inhibitors of α-glucosidase, and in addition, three of them demonstrated the ability to inhibit α-amylase.
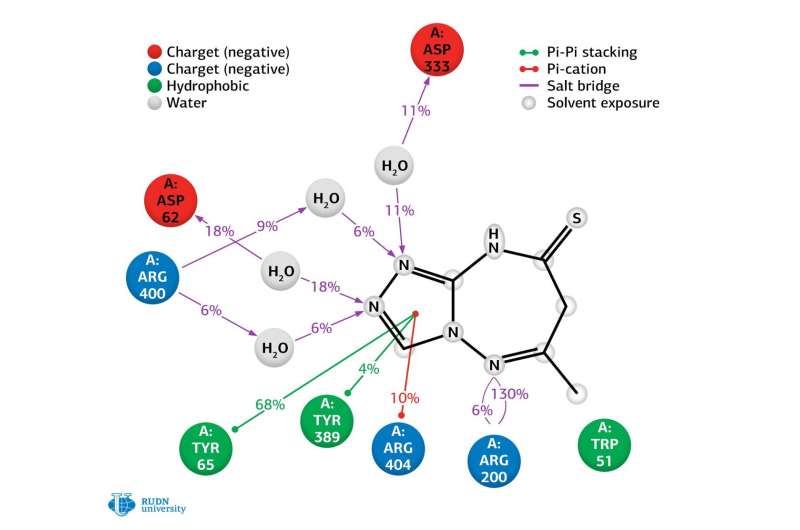
Using molecular docking, a method to model the properties of molecules, the researchers showed that three triazole derivatives—6—methyl-7H,8H,9H-[1,2,4]triazolo[4,3-b][1,2,4]triazepine-8-ones and 6-methyl-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazepine-8(9H)-tiones—outperform acarbose in inhibitory activity with respect to α-glucosidase.
Based on the results of the experiments, the chemists chose the best variant of the conformation of 6-methyl-7H - [1,2,4]triazolo[4,3-b] [1,2,4]triazepine-8 (9H)-thiones in complex with α-glucosidase and conducted its molecular dynamic modeling.
Calculations showed that the degree of inhibition of α-glucosidase depends mainly on the number and strength of bonds between the compound and the active sites of the enzyme. The obtained results led researchers to conclude that the synthesized compound is stable.
Youness El Bakri and his colleagues also showed antioxidant properties of the new compounds. Spectrophotometric study revealed the change in the optical density of solutions containing specifically colored free radicals (cation-radical ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonate) and radical DPPH (2,2-diphenyl-1-picrylhydrazyl), to which antioxidants were added. With these methods, chemists determined the antioxidant's ability to interact with ABTS and DPPH radicals. Also, the antioxidant activity of 1,2,4-triazole derivatives was estimated by evaluating the ability of antioxidant iron recovery.
The chemists concluded that the obtained 1,2,4-triazole derivatives are promising for use as antidiabetic drugs. They expect that the properties of these substances will soon become the subject of toxicological and pharmacological studies in vivo.
More information: Youness El Bakri et al. Synthesis, biological activity and molecular modeling of a new series of condensed 1,2,4-triazoles, Bioorganic Chemistry (2019). DOI: 10.1016/j.bioorg.2019.103193
Provided by RUDN University