January 6, 2020 feature
Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics
Materials scientists aim to enable surface-trap-mediated nonradiative charge recombination to engineer highly efficient metal-halide perovskite photovoltaics (solar cells). Since unproductive charge recombination at surface defects can limit the efficiency of hybrid perovskite solar cells, scientists can passivate the defects (induce an acid-base chemical treatment) using small molecular binding. The ionic character of perovskite lattice can allow molecular defect passivation through interactions between functional groups and surface defects. However, there exists a lack of in-depth understanding on how molecular configurations can influence passivation effectiveness to facilitate rational molecular design.
In a new report on Science, Rui Wang and an interdisciplinary research team in the departments of Physics, Materials Science & Engineering, Nanoengineering, Chemistry & Biochemistry and the Institute of Functional Nano & Soft Materials in the U.S. and China, investigated the chemical environment of a functional group activated for defect passivation. They conducted experiments to achieve enhanced power conversion efficiencies for perovskite photovoltaics using theophylline, caffeine and theobromine compounds bearing carbonyl (C=O) and amino groups (N-H). In theophylline treated experiments, hydrogen bonding of the amino hydrogen to surface iodide optimized the carbonyl interaction with a lead (Pb) antisite defect to improve the efficiency of a perovskite cell from 21 to 22.6 percent.
Materials scientists implement defect passivation as an important strategy to reduce unproductive charge recombination and increase power conversion efficiency (PCE) of polycrystalline metal-halide perovskite thin-film photovoltaics for solar cells. Based on Lewis acid-base chemistry, the ionic nature of the perovskite lattice can facilitate molecular passivation through coordinate binding. Based on molecular design rules, scientists can select molecules with optimal binding configurations for such surface defect passivation activities. In this work, Wang et al. demonstrated high efficiencies for perovskite (PV) devices via defect identification and conducted rational design and extensive investigations of the chemical environment surrounding the active functional group for defect passivation. In high-quality perovskite polycrystalline thin films with monolayered grains, the interior defects were negligible compared with surface defects.
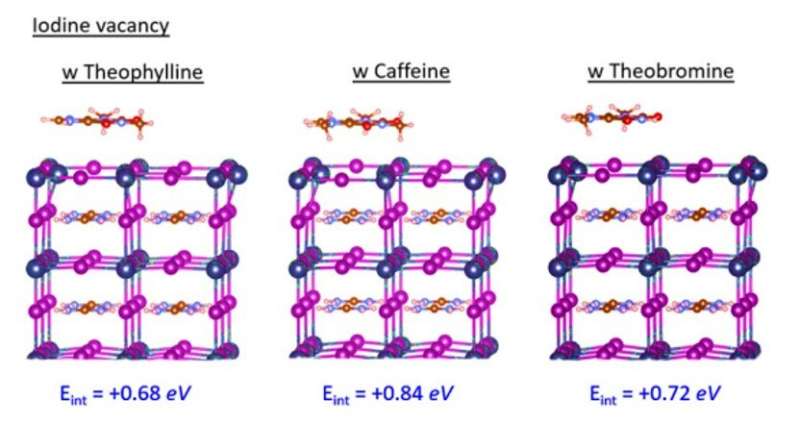
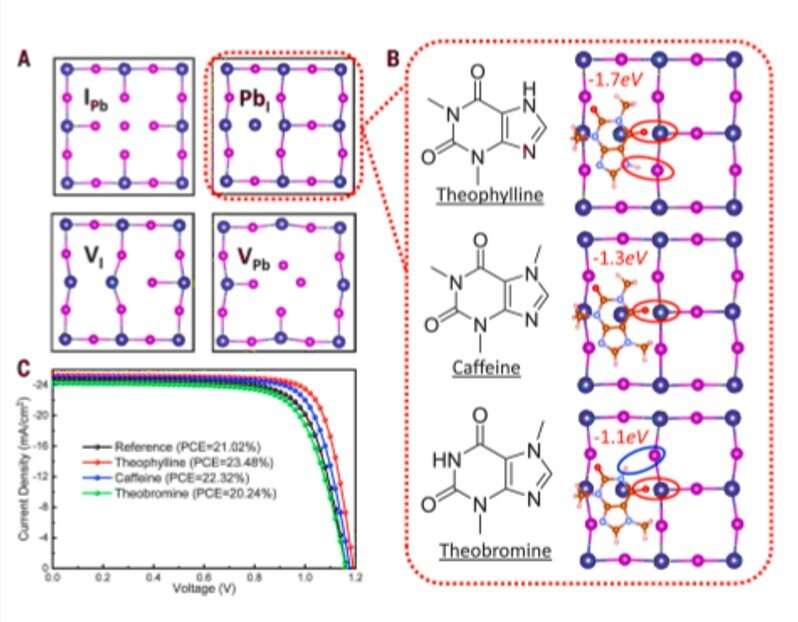
The research team used density functional theory (DFT) calculations to compare formation energies of selected native defects on the perovskite surface. Since band edges of the perovskites are composed of lead (Pb) and Iodine (I) orbitals, Wang et al. specifically investigated Pb and I-involving point defects, Pb vacancy (VPb), I vacancy (VI) and Pb-I antisite defects. Using X-ray photoelectron spectroscopy (XPS), the research team confirmed the surface of as-fabricated perovskite thin film to be synthesized in a two-step method to be Pb-rich. Then using the top-layer view of atomic structures, they studied surface defects, followed by the dispersion correction 3 (DFT-D3) method to calculate defect formation energies (DFE). Based on the results, the research team focused on the interaction between the surface Pb and antisite defect to consider candidate molecules for defect passivation. For this, they chose a small set of molecules that shared identical functional groups, although with strategically varying chemical structures to include theophylline, caffeine and theobromine, to interact with the defects.
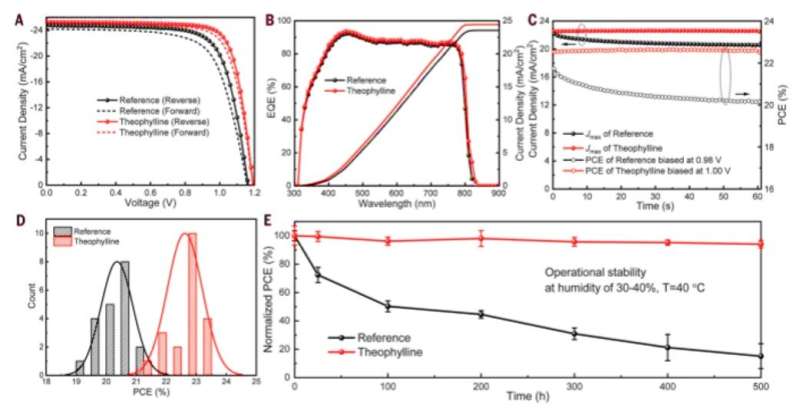
These molecules are typically found in natural products such as tea, coffee and chocolate, and are therefore readily accessible. The molecules were also nonvolatile in nature, rendering them suitable for interactions with defects in perovskite for long-term device stability. Wang et al. incorporated theophylline onto the surface of a perovskite thin film via a post-treatment technique to enhance the PCE (power conversion efficiency) from 21 percent to 23 percent in the PV devices. They tested the current density-voltage curves of the PV devices with and without theophylline treatment and credited enhanced open-circuit voltage (VOC) to surface passivation by theophylline due to Lewis base-acid interactions between the C=O group on theophylline and the antisite Pb surface defects. They then compared the results of a theophylline-treated device to a caffeine-treated perovskite PV device.
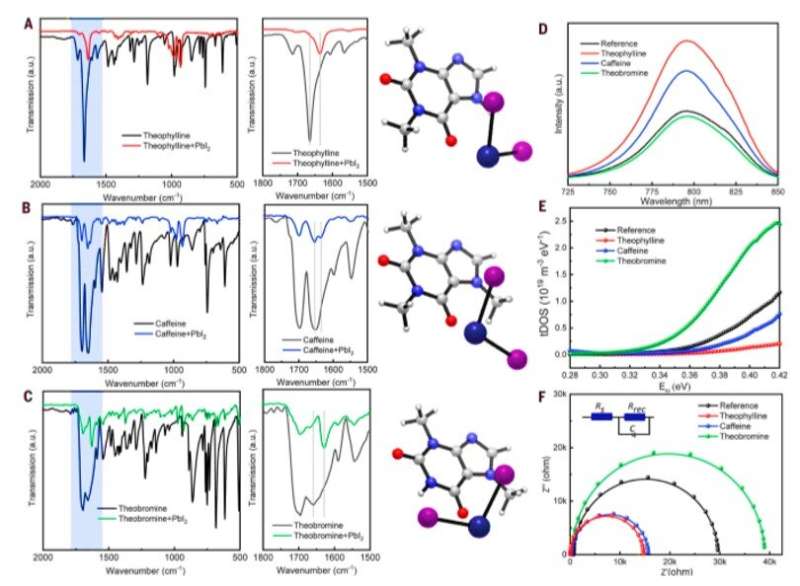
Subsequently, Wang et al. located the N-H group next to the C=O (carbonyl) group on the same six-membered ring in theobromine to produce a shorter distance between the two groups, followed by disabling spatially effective interactions to form an even weaker interaction energy (Eint) of -1.1 eV. The results emphasized the importance of constructive configuration of N-H and C=O groups to allow cooperative multisite interactions and allow synergistic passivation effect to form efficient and stable perovskites. Wang et al. studied the variation in the C=O and PbI2-terminated perovskite surface interaction with different configurations using Fourier-transform infrared (FTIR) spectroscopy. They examined surface passivation effects of the three molecules using different configurations with photoluminescence (PL) and observed PL intensity to noticeably increase after theophylline treatment. They also observed enhanced PL intensity after caffeine treatment, which was not as strong as theophylline and decreased PL intensity for theobromine compared with the reference material; they credited this to the destructive molecular configuration of passivation agents to produce increased charge recombination sites.
The scientists then deduced trap density of states (tDOS), i.e., the number of states occupied in the system, within as-fabricated devices via angular frequency-dependent capacitance as a function of the defect energy. The results demonstrated a reduction in trap states for theophylline and caffeine-treated perovskite devices compared with the reference material. In contrast, theobromine treatment induced more trap states, consistent with the observed decrease in PCE. Wang et al. confirmed the change in tDOS with different surface treatments using theoretical modelling and conducted electrochemical impedance spectroscopy (EIS) characterization to understand carrier transport processes under illumination at the interface.
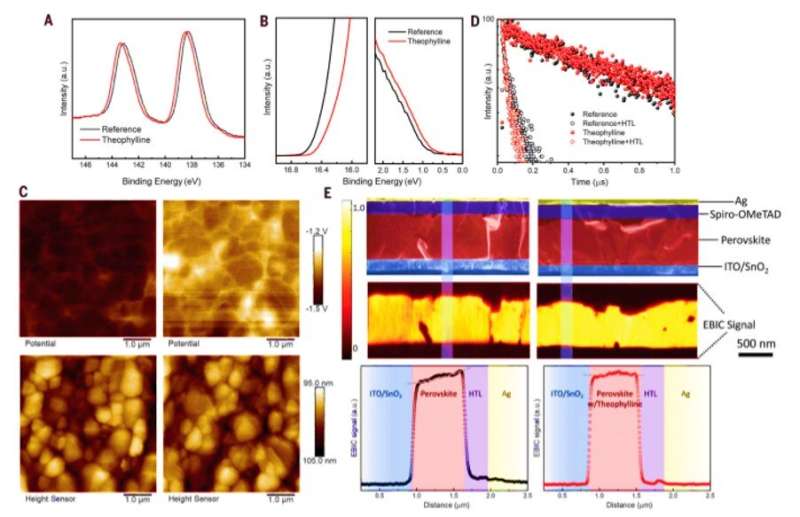
The device with theophylline surface treatment had the smallest impedance; signifying a substantially suppressed charge recombination at the interface, originating from reduced surface defect states. The caffeine-treated devices recorded a larger impedance while theobromine-treated devices demonstrated an even larger impedance. To understand perovskite interface treated with theophylline, the scientists conducted further characterizations using ultraviolet photoelectron spectroscopy (UPS) to measure the surface band structure. Followed by atomic force microscopy (AFM) combined with Kelvin probe force microscopy (KPFM) to understand the influence of theophylline on surface morphology and surface potential. The theophylline-treated surfaces exhibited higher electronic chemical potential compared to the reference film while retaining the unchanged surface morphology.
The perovskite film showed a slightly long carrier lifetime after theophylline treatment while observing a faster decay profile upon adding a hole-transporting layer on top of the film to reduce recombination and increase absorption properties. The improved carrier dynamics originated due to effective surface passivation with theophylline. When Wang et al. further characterized the surface using cross-sectional electron-beam-induced current (EBIC) measurements; theophylline treated devices exhibited higher EBIC current compared with the reference device to indicate enhanced carrier collection efficiency.
Theophylline treatment also allowed for minimal decay in the perovskite layers to result in fewer surface recombination sites and showed negligible hysteresis (microscopic surface defects). The enhanced shelf-stability of theophylline-treated devices could maintain >95 percent of its original PCE upon storage under ambient conditions of humidity for 60 days. In this way, Rui Wang and colleagues achieved stable power conversion efficiency for PV devices after incorporating theophylline for long-term operational stability.
More information: Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics, Science. December 2019. DOI: 10.1126/science.aay9698
Xiaopeng Zheng et al. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations, Nature Energy (2017). DOI: 10.1038/nenergy.2017.102
Qi Jiang et al. Surface passivation of perovskite film for efficient solar cells, Nature Photonics (2019). DOI: 10.1038/s41566-019-0398-2
Journal information: Science , Nature Energy , Nature Photonics
© 2020 Science X Network