Cell growth: Intricate network of potential new regulatory mechanisms has been decoded
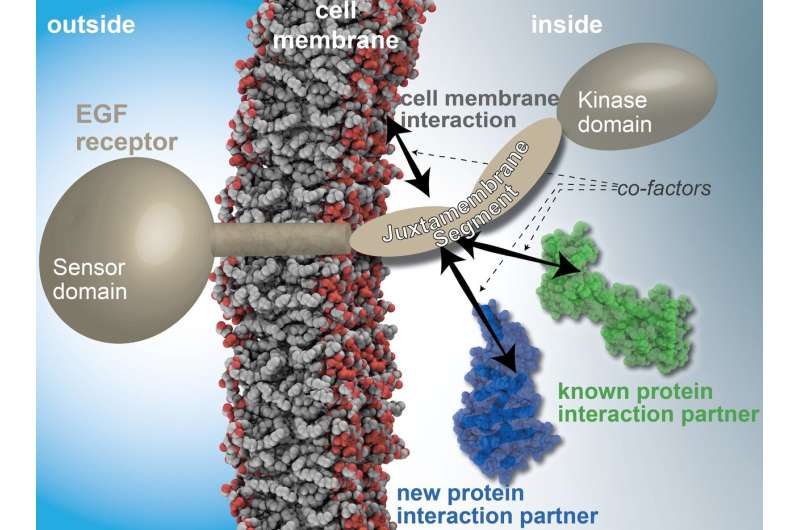
Whether a cell grows, divides or dies is controlled, among other things, by receptors that messenger substances bind to externally. A research team from Heinrich Heine University Düsseldorf (HHU) and Forschungszentrum Jülich (FZJ) worked together with partners from the University of Bonn to study the important EGF receptor in more detail. They succeeded in uncovering more information on an interface about which so far very little was known.
In the cover article of the Cell Press journal Structure, the authors—among them Dr. Manuel Etzkorn (HHU/FZJ) and Prof. Dr. Michael Famulok (Bonn)—now describe how the interface functions and what substances can interact with it.
Among the things that control cell growth are proteins in the cell membrane. In this regard, EGF receptors ("EGF' stands for Epidermal Growth Factor) form a central interface between the cell and its environment. This is why disruption to this system is a frequent cause of cancers, which arise from incorrectly controlled cell growth.
Many drugs have a direct impact on EGF receptors. These drugs work by focusing on two key areas: The first is the sensory domain, which reaches out of the cell and interacts directly with messenger substances that bind to the cell externally. The second is the kinase domain, which is located inside of the cell and transmits the signal. In certain cancers, the cells have already developed a resistance to active ingredients that target these two domains.
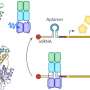
The EGF receptor additionally comprises one other domain: the juxtamembrane (JM) segment between the external sensory domain and the kinase domain. We know that molecules also interact with this segment and can influence the transmission of signals as a result. But so far we know of very few interaction partners. It is also unclear exactly how these interactions take place.
Researchers from HHU and FZJ as well as the University of Bonn have now identified a network of interaction partners for the JM segment. They also obtained high-resolution insights into the molecular architecture underlying the interaction. This means that receptor's third domain can now also become more significant for the development of new active ingredients. In particular, it offers a new therapeutic approach for cancers that have become resistant to current active ingredients.
"Our research findings are initially of a fundamental nature; they show new possibilities for influencing the EGF receptor system under defined laboratory conditions. This means that we are opening the door to developing new drugs, but there is still a very long way to go to a new therapy," says Dr. Manuel Etzkorn from the Biomolecular NMR Center, which is jointly run by the Institute of Physical Biology at HHU and the Institute of Complex Systems at FZJ.
In this newly published study, his team shed light on the aspects of this biological structure. The colleagues in Bonn under the leadership of Prof. Dr. Michael Famulok from the LIMES Institute and the Center of Advanced European Study and Research initiated the project and carried out the biochemical and molecular biological characterisation of the systems studied.
More information: Aldino Viegas et al, Molecular Architecture of a Network of Potential Intracellular EGFR Modulators: ARNO, CaM, Phospholipids, and the Juxtamembrane Segment, Structure (2019). DOI: 10.1016/j.str.2019.11.001
Journal information: Structure
Provided by Heinrich-Heine University Duesseldorf