Structures in seaweed shed light on sustainability
Brown algae are not just seaweed that float in the ocean and tangle swimmers' feet—they also contains a secret. In its cell wall, brown algae hold the polysaccharide alginate, one of the most abundant carbohydrates in the ocean. A major food source for several organisms, the alginate absorbs carbon dioxide and can be converted into ethanol.
Researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences are now studying the organisms to understand this mechanism, and they're making some discoveries along the way.
"The abundance of these seaweeds has made them an attractive and important source of renewable biomass for biofuel production," said Ji Shiqi, research assistant professor in the Shandong Provincial Key Laboratory of Energy Genetics.
Ji is part of an international team from China and the United Kingdom working to better understand how the alginate is processed into ethanol by organisms. By examining the enzymes that break down the alginate, the researchers may be able to harness the natural process to produce biofuel. During this process, they identified previously unknown enzymatic families that contribute to the bioconversion.
Their findings about the full structure of one such enzyme were published in Journal of Biological Chemistry on October 17.
The enzyme, called an alginate lyase, breaks down the alginate so it can be converted into other products. There are currently 37 identified lyase families that break down polysaccharides, which are structures that contain multiple sugars. Of those 37 families, nine specifically degrade alginates and seven of those nine have had their structures fully described.
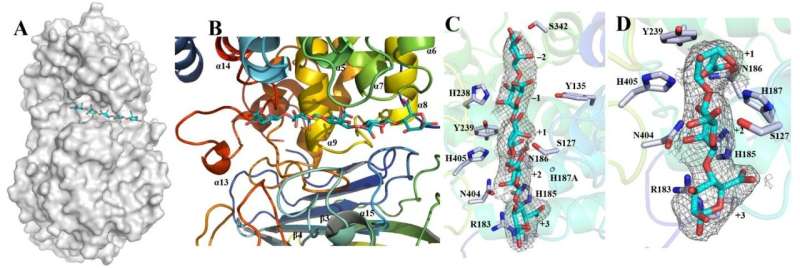
Ji and the team identified a new alginate lyase in a heat-loving bacterium that can directly utilize brown algae and ferment its components to ethanol with high-yield.
"The bacterium contains at least four alginate lyases, including a number of novel lyases that represent totally new polysaccharide lyase families," Ji said.
The researchers focused on imaging the structure of one alginate lyase, dubbed "Dp0100," to better understand its molecular mechanisms in processing alginate into ethanol. The imaging study also led to a better understanding of the structure specificity, catalysis and evolution of alginate lyases with multiple domain sites, according to Ji.
"While the mechanism of Dp0100's thermostability is not well understood yet, this research has furthered our understanding of the structure-function and evolutionary relationships within this important class of lyases," Ji said.
The researchers will continue to study the alginate lyases, as well as pursue structural studies of other polysaccharide lyase families with the ultimate goal of determining the full function of alginate bioconversion.
More information: Shiqi Ji et al, The molecular basis of endolytic activity of a multidomain alginate lyase from Defluviitalea phaphyphila, a representative of a new lyase family, PL39, Journal of Biological Chemistry (2019). DOI: 10.1074/jbc.RA119.010716
Journal information: Journal of Biological Chemistry
Provided by Chinese Academy of Sciences