A more efficient way to turn saltwater into drinking water
Water scarcity is a major problem across the world. "It affects every continent," says Amir Barati Farimani, an assistant professor of mechanical engineering at Carnegie Mellon University. "Four billion people live under conditions of severe water scarcity at least one month of the year. Half a billion people live under severe water scarcity all year."
Yet even as people struggle without access to safe drinking water, there are oceans of undrinkable water right outside their doors. "71% of the world's surface is covered by seawater," Barati Farimani says. "So this is a very interesting contradiction."
In order to combat this problem, Barati Farimani has focused his research on water desalination. This is the process in which salty seawater can be transformed into fresh water.
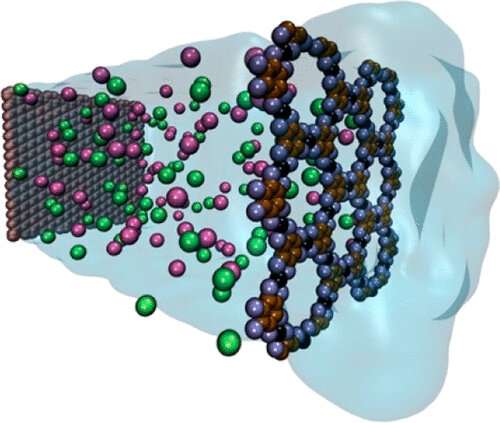
There are many ways to desalinate water, but one of the most effective is membrane desalination. In this method, water is pushed through a thin membrane with tiny holes. The water flows through the pores, but the salt ions can't, leaving only fresh water on the other side.
In his latest research, Barati Farimani explores the potential of a new type of membrane, called a metal-organic framework (MOF). "These membranes consist of both the metal center and organic compound," Barati Farimani says. The organic compound and metal connect in a pentagonal pattern, leaving a hole in the center that serves as a pore. "If you look at them, they are like a honeycomb," Barati Farimani adds.
There are a couple of reasons why the framework is more effective. First, it's incredibly thin. It's a few atoms thick, which means there's very little friction as the water molecules pass through the pores.
Additionally, the placement of the pores helps with permeation. "When you don't have adjacent pores, there's a huge pressure from the wall on the molecules," Barati Farimani says. This makes the desalination process less efficient. To understand why, just imagine pouring water into a funnel. The water moves more slowly through the hole at the end because it's pushed against the walls and forced through a small space.
The MOF, on the other hand, has multiple adjacent pores. "There's no pressure from the wall side," Barati Farimani says. "And that gives them this opportunity to pass more easily through the pore." Imagine pouring water through a strainer this time—it moves much more quickly, because it has multiple exit points it can escape through.
Finally, the MOF has more structural integrity than other materials. In most materials, scientists have to drill tiny holes in order to create the needed pores, which limits the amount that can be created per surface area. "If you want to make a lot of pores, graphene or MoS2 can't do that," Barati Farimani says. "Structurally they can't hold the pressure."
But thanks to its honeycomb structure, MOF is intrinsically porous. This allows a higher ratio of pores to surface area. It also saves on time and energy, since the pores don't need to be drilled, or even adjusted in size.
The differences between the MOF and other typical membranes are notable, both in terms of how quickly water passes through and how many ions are rejected. And that's just looking at a simulation of a few pores. A desalination plant can have billions of pores, raising its efficiency exponentially. "In the scale of a large operation, it would be huge," Barati Farimani says. "Even a slight increase in efficiency would mean a huge leap."
Barati Farimani's article on his research was published in Nano Letters, a monthly peer-reviewed scientific journal published by the American Chemical Society. It adds to a growing conversation about water desalination and represents an important step forward in the field.
In addition to the world of academics, Barati Farimani hopes that his research can make an impact in people's lives. "We need to provide fresh water for many underprivileged people, like in Africa or other places," he says. "Basically that's our mission—to make it so energy efficient that we have water desalination everywhere."
More information: Water Desalination with Two-Dimensional Metal–Organic Framework Membranes, ACS Nano, pubs.acs.org/doi/10.1021/acs.nanolett.9b03225
Journal information: Nano Letters , ACS Nano