November 22, 2019 report
Physicists determine dripline for fluorine and neon isotopes
An international team of physicists with the BigRIPS experiment taking place at the RIKEN Radioactive Isotope Beam Factory in Japan has determined the dripline for fluorine and neon isotopes. In their paper published in the journal Physical Review Letters, the researchers describe how they found the driplines and where their research is headed next.
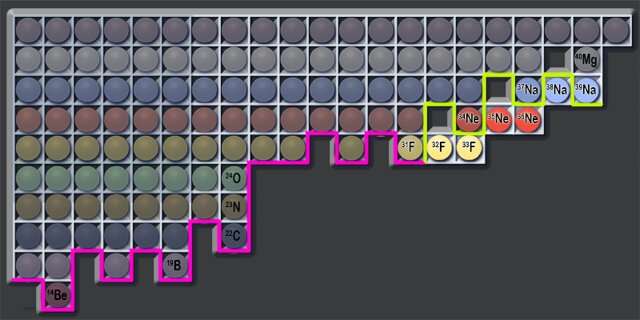
One of the goals of physics research is to discover some of nature's limits—in this new effort, the researchers were looking to determine how many neutrons could be added to a nucleus before they stop sticking to one another, leading to dripping out. Such a limit is known as the dripline. Prior researchers have already found the dripline for a number of elements—all eight of the lightest of them, for example. Doing so for the heavier elements has been challenging. In this new effort, the researchers sought to find the dripline for fluorine, sodium and neon. Prior research has shown that adding neutrons to a nucleus increases its atomic number when the maximum is found—but there are exceptions. The driplines for isotopes such as nitrogen-23, carbon-22 and oxygen-24 are all 16 neutrons, for example.
To find the dripline for the target elements, the researchers focused beams of calcium-48 ions at beryllium atoms, resulting in fragmentation and the creation of small nuclei. They studied the fragments using the BigRIPs large acceptance fragment separator—a two-stage process whereby a primary beam is first converted to radioactive ion beams by a uranium beam. That was followed by a tagging stage in which a secondary beam was tagged using the radioactive ions downstream.
The researchers report that they were unable to find any evidence of neon-36, neon-35, fluorine-33 or fluorine-32. They claim this showed evidence indicating that fluorine-31 and neon-34 are both dripline isotopes. They report that they also looked for sodium-38 and 39 and found one instance of sodium-39 but no sodium-38—they suggest this likely indicates that the dripline for sodium isotopes is beyond 28 neutrons.
The researchers note that in 2022, a new facility will open at Michigan State University with more intense beams, giving researchers a chance to discover the dripline for sodium and then to keep working along the periodic table to resolve those next in line—starting with magnesium.
More information: D. S. Ahn et al. Location of the Neutron Dripline at Fluorine and Neon, Physical Review Letters (2019). DOI: 10.1103/PhysRevLett.123.212501
Journal information: Physical Review Letters
© 2019 Science X Network