The similarities between a Van Gogh painting and a golf ball

On a molecular scale, there are surprising similarities between the outer shell of a golf ball and the white oil paint used by Van Gogh and his contemporaries. In both cases, the interactions between zinc ions and polymer chains are at the basis of important material properties. In a recent publication in the scientific journal Science Advances, Rijksmuseum and University of Amsterdam researchers describe the role of zinc ions in the molecular network of oil paint. Their studies could explain why paintings made with zinc white pigment are so sensitive to high humidity conditions.
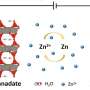
Vincent van Gogh and other painters of his day often used zinc white (zinc oxide, ZnO), a white pigment that yields a good opaque oil paint. However, zinc white can easily react with the oil binder as it polymerises and transform into a network of molecular chains during paint drying. As a consequence, zinc ions nestle themselves between the molecular chains of the oil. Previous research showed that this process is not without danger—the ions can speed up the breakdown of the oil, and they form problematic chemical compounds with the degradation products.
Golf balls
To help conservators minimise damage and slow down such oil paint aging, Dr. Joen Hermans and colleagues have now resolved the molecular structure around the zinc ions in oil paint. The research has also yielded important insights in the world of plastics. Some plastics contain zinc ions so they can be melted and processed at lower temperatures, while still being strong and durable. Therefore, this type of plastic is very suitable for the outer shell of golf balls, for example. Researchers had not yet determined exactly how the zinc ions keep the polymer chains together in these plastics.
The researchers of the University of Amsterdam and the Rijksmuseum have now answered these questions about the polymer environment around zinc ions in an advanced analysis combining infrared light with quantum mechanical calculations. They used two dimensional infrared spectroscopy (2-D-IR), a complex technique that is capable of measuring the interactions between chemical bonds and their orientation in space. 2-D-IR was never before used to study molecular structure in solid polymers.
The role of water
The research demonstrated that the polymer surroundings of zinc ions can adopt two completely different structures. The exact same two structures exist for the zinc ions in the plastic outer shell of golf balls and similar materials. Moreover, the researchers discovered that very small amounts of water in an oil paint or plastic determine which of the two surroundings is more likely to occur. Given that one of the structures is more active in the chemical reactions that cause paint ageing, this research yields a potential molecular explanation for the fact that oil paintings made with zinc white tend to age faster at high humidity conditions.
These new insights are very important for follow-up research on the effect of humidity on the lifespan of a painting. Additionally, they are a starting point to determine which paintings will be most sensitive to future alterations. Researchers at the Rijksmuseum and the University of Amsterdam will continue this research in the next few years.
More information: Joen. J. Hermans et al. 2D-IR spectroscopy for oil paint conservation: Elucidating the water-sensitive structure of zinc carboxylate clusters in ionomers, Science Advances (2019). DOI: 10.1126/sciadv.aaw3592
Journal information: Science Advances
Provided by University of Amsterdam