Modeling Earth's chemistry: Making the invisible visible
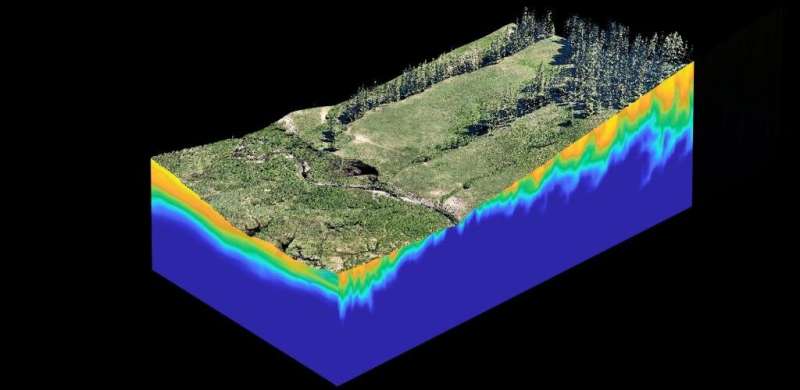
An incredibly complex system lives beneath our feet, transporting metals to Earth's crust and undergoing a myriad of chemical reactions that influence our daily lives. These environmental interactions affect everything from our ability to use soil to produce food and the cleanliness of our drinking water to how we might mitigate our changing climate. Humans have a huge impact on Earth's subsurface—through mining, fossil fuel extraction, irrigation and energy waste storage—and we have to deal with the environmental problems that ensue. And yet, we can't see it.
To virtually peer into the ground, many researchers use complex modeling approaches that account for factors like the interactions among microorganisms and how plants absorb and return water and nutrients. These biogeochemical approaches—essential tools for the Earth sciences and other fields—are the bread and butter of research by Kate Maher, an associate professor of Earth system science at Stanford's School of Earth, Energy & Environmental Sciences (Stanford Earth).
In this Q&A, Maher explains how modern scientists make the invisible visible as they investigate the processes that transport contaminants and shape Earth's surface. To do this, they use modeling and visualizations that incorporate the latest mathematical techniques, sensing technology and massive amounts of data. Maher co-edited the current special issue of Elements Magazine, titled "Reactive Transport Modeling," that provides a more in-depth look at this field.
What is reactive transport modeling?
Most of the water we drink spends a considerable part of its life as groundwater. As water flows through the ground, it interacts with complex mineral surfaces, organic matter and microorganisms that can ultimately influence how nutrients and contaminants are transported through environmental systems. Groundwater systems extend from meters to kilometers below the surface, and thus models are the only tool we have to study the invisible life of water.
Reactive transport models (RTMs) are advanced algorithms that combine descriptions of fluid flow, transport processes and biogeochemical reactions in order to calculate changes in solutes, minerals and even microbial communities over space and time. The models have been built up over decades to continually incorporate state-of-the-art descriptions of the transport processes as well as the biogeochemistry. In some sense, they are a library containing our knowledge of everything from the physics of groundwater flow to the details of microbial metabolisms.
The reactions and the transport have to be computed together because they often strongly interact, and this is especially important for systems that have been impacted by human activity. For example, at many sites affected by groundwater contamination, a common method to clean up the water is to inject organic carbon to cause a reaction in the microorganisms. But the attempt could fail if there is too much microbial growth near the well, clogging the pore space. By using models to simulate a cleanup strategy, scientists at these sites can design better strategies to clean up the water.
The ability to model processes over long time-scales, or even thousands to millions of years, is another key feature of RTMs. These models have helped us understand the rate at which rocks dissolve to form soils, or the components of chemical weathering—from the role of plants and microorganisms in dissolving minerals to the rate at which carbon dioxide in rainwater is converted into bicarbonate, a key process in the long-term carbon cycle that controls our atmosphere.

What are some applications of reactive transport modeling?
Most of the landscapes we see around us contain a legacy of the past that can be critical to understanding the human-driven or natural perturbations occurring today and into the future. Sometimes geoscientists discover puzzling signals in ancient rocks and want to know what they might tell us about Earth's environments millions to billions of years in the past. Given the need to span a diverse array of time scales and processes, reactive transport has found its way into nearly every single field of the geosciences and we give some examples in our introductory article, followed by six other topical articles.
Nuclear waste storage has been a very important application, given the need to predict the stability of various waste packages for hundreds of thousands of years under unknown future climate scenarios. Groundwater contamination has been another key area. Environmental cleanup strategies, especially those involving microorganisms or other engineered interventions, need to be simulated and understood for each site before they are deployed. At contaminated sites, RTMs are used both as tools for scoping cleanup strategies and to develop regulatory guidelines. One important example has been the use of models to understand arsenic contamination at sites around the world. Finally, geologic carbon storage, which involves injection of massive amounts of carbon dioxide into deep geologic layers, has been another area where models are used to estimate how much of the carbon dioxide dissolves in the groundwater and how much might become insoluble, and therefore more permanently sequestered.
How can these techniques inform our understanding of climate change or achievement of climate solutions?
Humans are injecting carbon into the ocean-atmosphere system at a rate that is about 70 times Earth's capacity to sequester it. Earth sequesters carbon through a sequence of reactions involving dissolution of minerals in soils followed by precipitation of limestone in the oceans. A key question is: How might we mimic this natural process to safely store the carbon dioxide that we emit? In some rocks, the carbon dioxide will never form minerals and it will always have the potential to migrate into drinking water supplies or back to the atmosphere.
Soils are another key area. Soil carbon is the largest reservoir of carbon at or near Earth's surface and thus it is especially sensitive to land-use changes, as well as to changes in temperature and soil moisture associated with climate change. Many of the current Earth systems models that are used to predict the carbon cycle into the future—including those used by the Intergovernmental Panel on Climate Change (IPCC) – contain outdated descriptions of soil carbon transformations and no explicit treatment of microorganisms. To address this problem, scientists using RTMs are actively engaged in finding ways to improve the representation in soil carbon in Earth system models. This can range from the effect of drought on microorganisms to the role that soil minerals play in sequestering carbon. The ultimate goal is to reduce the uncertainty surrounding the response of soils to climate change.
What made you pursue this field and what skills does it require?
As an undergraduate, I always loved computer science. However, having grown up in the mountains of the West, I was also deeply concerned about the environment. One of my first courses in graduate school was in geodynamics, and in looking for a topic for my final paper, I discovered reactive transport models and was completely fascinated.
I would say the most important skill is probably the ability to learn from others. RTMs draw on the knowledge and expertise of an incredibly diverse array of fields, such that there are very few people who can fully understand both the numerical and conceptual underpinnings of the models. There will always be someone who knows more about the history of the field site, the microbial metabolisms or the linear algebra libraries. The job of the modeler is ultimately to harvest this information in meaningful ways. However, the endless potential to integrate knowledge across scientific communities also means that RTMs can be incredibly powerful platforms for collaboration.
I have learned that a blend of determination, curiosity and patience is critical. In our toolkit article, we outline some of the key areas. A solid background in programming and math are extremely useful, or at least make it easier to get started. The models are so complex that it is easy to produce results that don't make physical sense, so the ability to use the governing equations for mass, momentum and energy conservation to develop limiting cases is also extremely important.
Most of the mainstream RTMs are built and maintained by U.S. Department of Energy National Laboratory scientists, which means that there are relatively few training opportunities and the field is still small, with only a few programs at universities. Given the vast potential of these models, this is something scientists using RTMs are trying to address by developing innovative new opportunities for education.
Provided by Stanford University