June 10, 2019 feature
Biotechnology: Using wireless power to light up tiny neural stimulators
""
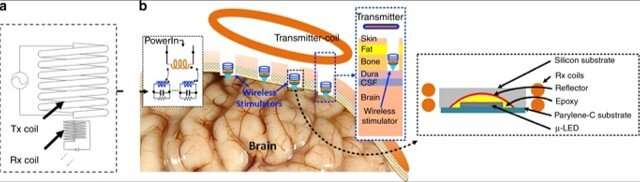
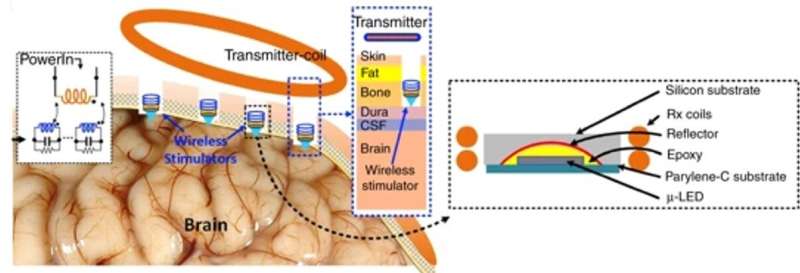
For this, the team coupled a microscale light-emitting diode (LED) with two millimeter-scale coils to create an inductive charging system, which delivered instantaneous power at biologically safe frequencies in an experimental rodent (rat) model. The wireless setup stimulated neurons in the visual cortex, while maintaining the temperature increase below 10C as a critical safety threshold for biomedical implants. The results are now published on Microsystems and Nanoengineering.
Khan et al. introduced a single channel neurostimulator containing a reflector-coupled, microscale light-emitting diode (µLED) with an integrated millimeter sized wireless receiver (RX) coil. The experimental setup allowed for free-floating, battery-free, untethered optogenetics neuromodulation. They used a two-coil inductive link in the system to deliver instantaneous power at a low operating frequency (<100 MHz) for continuous optical stimulation.
The process posed minimal invasiveness and tissue exposure to electromagnetic radiation. When they coupled a microscale reflector to the µLED, the optical-reflector displayed significantly enhanced light intensity compared to bare µLED. The scientists controlled the operational temperature of the setup for implant biocompatibility and conducted in vivo experiments in rats, followed by histological studies to verify the efficacy of wireless optical stimulation in the primary visual cortex of the animal model. They visualized the process using c-Fos biomarker, which appeared green on immunostaining, as a reporter of light-evoked neuronal activity.
Discoveries and inventions in neuroscience have recently progressed rapidly due to advances in semiconductor implants in neurobiological systems, for successful clinical translation. For example, scientists can use implantable "electroceuticals" in a new medical approach to target the central and peripheral nervous system during therapeutic intervention. As a result, optogenetics is finding new applications in neuroscience to deliver light to neural tissues of interest while collecting readouts from cells using targeted control tools.
The ability to implant miniature optical sources, recording electrodes, sensors and other components in to designated areas of the brain has renewed the optimism for long-term diagnostics and therapeutics. Using such developments, scientists can study the transmission of primary sensory information to specific domains of the brain, including the olfactory, visual and auditory regions in depth. They can also use the technique to understand cells that drive or inhibit fundamental bioactivities such as hunger, thirst, energy balance and respiration via activity patterns.
Optogenetics must target the neural population without altering the natural behavior of animal models for accurate applications in medicine. Pioneering work by researchers in the field have led to the development of several optimized neuro-stimulators with mid-field radiofrequency (RF) and far-field power transfer. However, scientists are yet to report on a fully implantable and miniaturized high voltage (HV) stimulator that provides precise stimulation control of the parameters of interest. An ideal wireless optical implant must therefore:
- Be miniature in the millimeter scale (mm) to prevent invasive surgical infection, inflammation and post-surgical trauma.
- Allow highly efficient power transfer and long-distance communication to deep region target neurons with aims for human applications.
A significant challenge with engineering such implants is the energy required to optically activate optogenetic opsins, which typically include a few mWs, although greater than the values for conventional electrical stimulation or data communication. To solve this challenge, Khan and co-workers proposed a fully implantable, mini wireless optical stimulator to deliver sufficient power for µLED operations—without surpassing the operational temperature. In the proposed system, they included a solenoid transmitter (TX) coil and receiver (RX) unit.
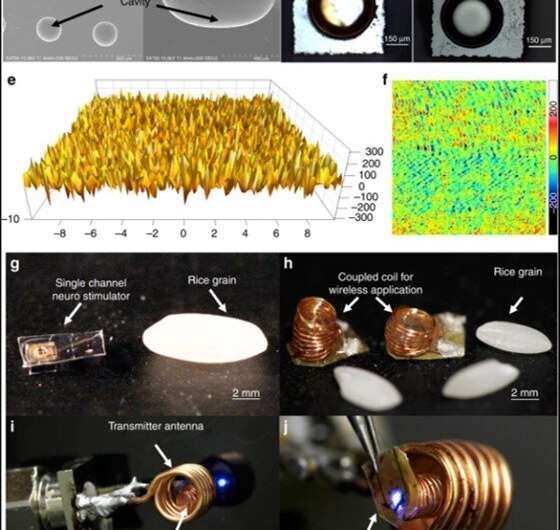
In a proof-of-concept of the proposed prototype, Khan et al. used a blue µLED (465 nm wavelength) of 270 µm x 220 µm surface area to optically excite neurons expressing channelrhodopsin. To validate the functionality of the device in an animal model, the scientists placed the Tx coil outside the brain and inductively coupled it to the Rx coil, which was integrated to the µLED neurostimulator placed within a craniotomy cavity. The scientists used the free-floating method for epidural optical neuromodulation in the craniotomy cavity located on top of the dura mater.
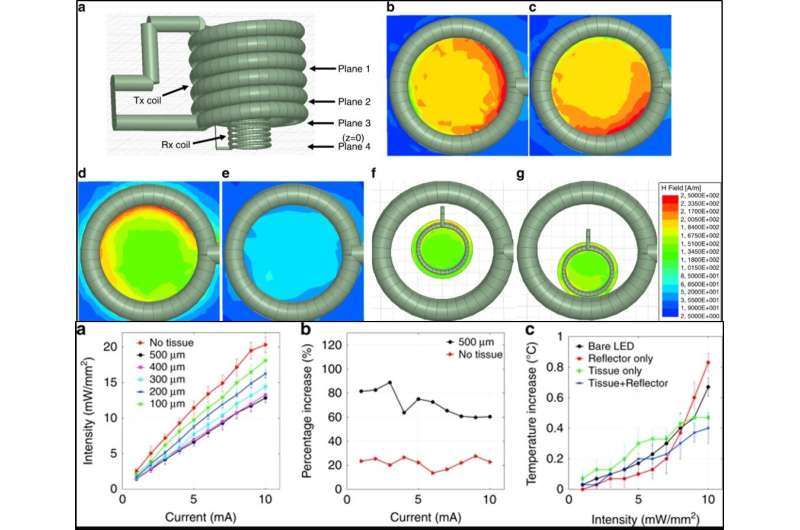
The scientists tested the surface morphology of the prototype using scanning electron microscopy to observe the cavity array after isotropic silicon etching and surface aluminum coating. They then tested the surface roughness using atomic force microscopy to detect surfaces that created negligible light scattering for optimally enhanced light intensity and activated the neurostimulator on a benchtop setup via inductive powering. The scientists also incorporated Parylene-c to engineer the constructs due to inherent biocompatibility of the material, although they observed potential cracking as before as a result of high temperatures in the engineering process.
Khan et al. simulated electromagnetic properties of the inductive link between the Rx and Tx coils using the finite element method (FEM) and high frequency structure stimulator (HFSS) software; to show similarities between the simulated Tx coil and those engineered in the work. The scientists investigated the optical properties and showed that the neurostimulator could reach deep brain cells less invasively compared to a waveguide or penetrating probes. While the data showed superior optical performance of the reflector-coupled stimulator, the intensity reduced heavily with thicker tissue sections. The intensity of the reflector-coupled stimulator was significantly higher in the present work compared with a bare stimulator for effective deep brain stimulation without deep-brain tissue invasion. Khan et al. similarly tested and optimized the thermal properties, electromagnetic properties and the power transfer efficacy of the neurostimulator prototype. To conduct translational studies in an animal model, the scientists proposed implanting the resonator coil between the skull and skin without wire connections to the Tx or Rx coil.
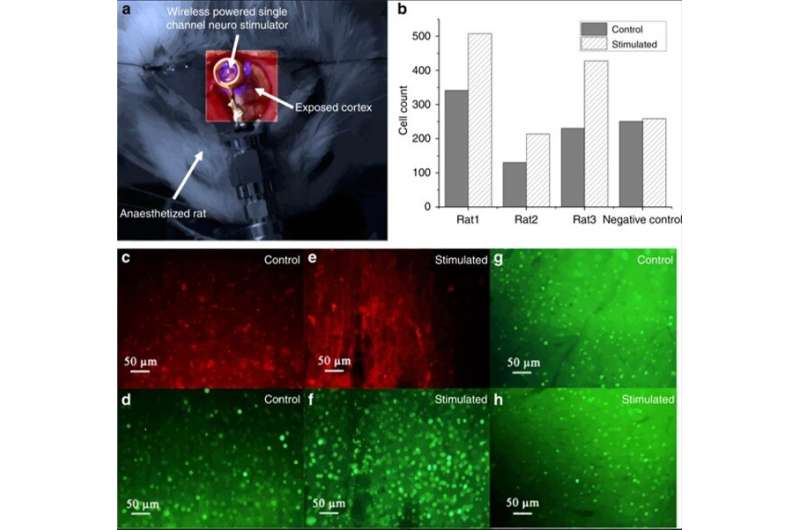
The scientists then conducted in vivo experiments in transfected rats (foreign DNA introduced into cells) to then implement the proposed surgical procedures. They followed the experiments with immunohistochemical assays to validate the efficacy of the cell transfection procedure and the subsequent optical stimulation procedure in the rats. During transfection, they induced the expression of channelrhodopsin-2 in the animal models with a viral solution and then placed the coiled stimulator on the primary visual cortex (V1 lobe) of the animal for subsequent optical stimulation. The scientists coupled the coiled stimulator to a Tx coil and used the other V1 lobe of the same animal as a control sample.
After completing the in vivo experiments, the scientists analyzed the expression of c-Fos (green dye) in the stimulated vs. non-stimulated lobe to identify neural activity. For this, Khan et al. used immunobiological assays in the work and observed elevated c-Fos expression (green) within the virus-transfected cortex (red) induced by LED stimulation of the experimental neurostimulator.
In this way, Khan et al. designed, fabricated and characterized a reflector-coupled, wireless single-channel optical neurostimulator with a mm-sized receiver coil for optogenetic neuromodulation. The reflector-coupled stimulator allowed higher performance compared to bare µLED in the present work. The scientists studied the performance of the two-coil telemetry link using analytical circuit models, FEM stimulations and experimental approaches. They verified the proposed potential of the neurostimulator in a rat model with upregulated cellular activity observed via optical stimulation. Khan et al. will aim to conduct further investigations in the future to miniaturize the devices for neurobiological applications.
More information: Wasif Khan et al. Inductively coupled, mm-sized, single channel optical neuro-stimulator with intensity enhancer, Microsystems & Nanoengineering (2019). DOI: 10.1038/s41378-019-0061-6
Kristoffer Famm et al. A jump-start for electroceuticals, Nature (2013). DOI: 10.1038/496159a
Karl Deisseroth. Optogenetics, Nature Methods (2010). DOI: 10.1038/nmeth.f.324
T.-i. Kim et al. Injectable, Cellular-Scale Optoelectronics with Applications for Wireless Optogenetics, Science (2013). DOI: 10.1126/science.1232437
Journal information: Nature , Nature Methods , Science
© 2019 Science X Network