Fuel cells in bacteria
The exchange of nitrogen between the atmosphere and organic matter is crucial for life on Earth because nitrogen is a major component of essential molecules such as proteins and DNA. One major route for this exchange, discovered only in the 1990s, is the anammox pathway found in certain bacteria. It proceeds via hydrazine, a highly reactive substance used by humans as a rocket fuel. Researchers at the Max Planck Institute for Medical Research, in cooperation with scientists from the Max Planck Institute for Biophysics and Radboud University in the Netherlands, now describe the structure of the enzyme performing the last step in this process: turning hydrazine into nitrogen gas and harvesting the energy set free in this way. The results, which were just published in Science Advances, show an unprecedented network of heme groups for handling the large number of electrons released during the chemical conversion.
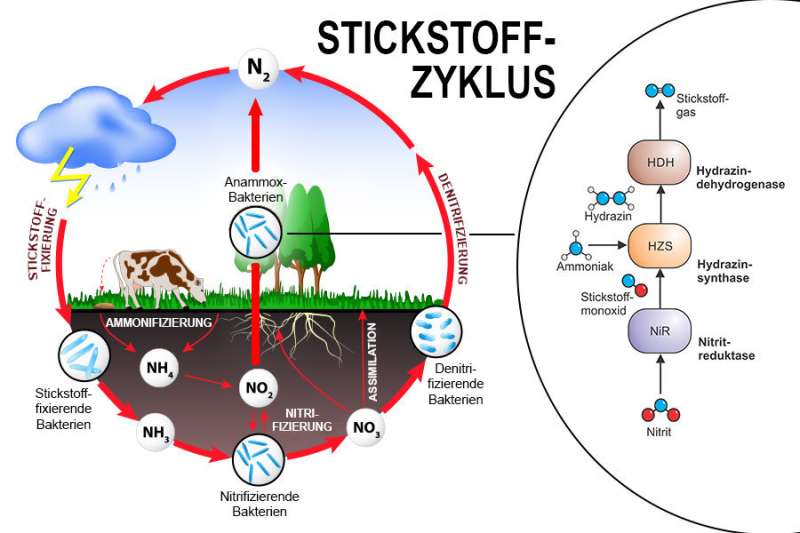
The biogeochemical nitrogen cycle
Nitrogen, in the form of nitrogen gas (N2), makes up about 80 percent of our atmosphere, but as an element nitrogen occurs only in small quantities in the Earth's crust. However, all living organisms require nitrogen, because it is part of most of their essential molecules. However, they cannot use atmospheric nitrogen directly and require it in a different chemical form. A number of bacteria perform such conversions and contribute to the biochemical nitrogen cycle (image) by producing more reactive forms of nitrogen.
Anammox Bacteria – a shortcut through the middle
In the 1990s, scientists discovered a bacterial process called anaerobic ammonium oxidation (anammox). "We now believe this process is responsible for 30 to 70 percent of the yearly nitrogen removal from the oceans," explains Thomas Barends, group leader at the MPI for Medical Research in Heidelberg. "Due to this characteristic, anammox bacteria are used in sustainable wastewater treatment all over the world," Cornelia Welte of Radboud University adds. During this process, bacteria convert nitrites and ammonia into dinitrogen (N2) and water, while generating energy for the cell. The molecule hydrazine is produced in an intermediate step. Hydrazine is a common component of rocket fuel, but its use by bacteria as a metabolic fuel is rather exotic—and surprising in living organisms because of its high toxicity. Welte: "So far, hydrazine has only been found in anammox and not in other bacteria." Until recently, little was known about how these bacteria harness the energy released during the hydrazine conversion.
Previously the research group and their collaborators have described the structures of the enzymes hydrazine synthase and hydroxylamine oxidoreductase. The researchers now further unravel the anammox puzzle by describing the crystal structure of hydrazine dehydrogenase, the enzyme involved in the conversion of toxic hydrazine to harmless dinitrogen gas. "Both the use of hydrazine as well as the structure of hydrazine dehydrogenase are quite unique, making it important to uncover the biological process in detail," Welte explains.
From toxic rocket fuel to harmless nitrogen – the hydrazine dehydrogenase (HDH) complex
"One could compare the HDH complex to a fuel cell with electrical outlets that only fit certain types of plugs," says Thomas Barends, describing the structure and mechanism of HDH. The 'fuel' hydrazine enters the protein complex through a channel on the outside. The enzyme then catalyzes the conversion of hydrazine into nitrogen gas through an unprecedentedly large network of 192 heme groups. Then the electrons are carried to other parts of the bacterium, like the transfer of current to electrical consumers. These consumers then generate the cell's energy.
Closing the gap
"We are now working on finding the protein that takes up the electrons stored in the heme network," says Mohd Akram, postdoc in the Barends group and first author of the paper. From the structure they observed they expect that only small proteins can enter the complex, take up the electrons in a hollow space inside, and leave again. Selecting which proteins can access the electrons may help ensure the electrons are brought to the right place to be used for energy generation in the cell.
More information: M. Akram et al. A 192-heme electron transfer network in the hydrazine dehydrogenase complex, Science Advances (2019). DOI: 10.1126/sciadv.aav4310
Journal information: Science Advances
Provided by Max Planck Society