February 22, 2019 report
A way to use a two-nickel catalyst to synthesize cyclopentenes
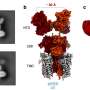
![Reaction development. (A) Complementary cycloaddition routes to five- and six-membered rings from 1,3-dienes. (B) Pericyclic [4 + 1]-cycloadditions suffer from large electronic barriers due to repulsion between the carbene lone pair and the Ψ1 orbital of the 1,3-diene. (C) Dinickel-catalyzed reductive [4 + 1]-cycloaddition of 1,1-dichloroalkenes and 1,3-dienes. NMP, N-methyl-2-pyrrolidone; rt, room temperature; c-Pent, cyclopentyl. Credit: (c) Science (2019). DOI: 10.1126/science.aau0364 **A way to use a two-nickel catalyst to synthesize cyclopentenes](https://scx1.b-cdn.net/csz/news/800a/2019/5c6fe6dca8c4e.jpg)
A pair of researchers at Purdue University has found a way to use a diatomic Ni-Ni catalyst to synthesize cyclopentenes. In their paper published in the journal Science, You-Yun Zhou and Christopher Uyeda describe their method and outline why they believe cyclopentene products would be useful. Keywan Johnson and Daniel Weix with the University of Wisconsin have published a Perspective piece in the same journal issue describing the work done by the team in Indiana.
Johnson and Weix note that the discovery of new molecules lies behind many of the new materials that scientists have created over the years. One of the ways that new molecules are discovered is by observing them in nature and then synthesizing them in a lab. They note also that transition metal catalysis has been widely used to synthesize many new molecules that are currently used in a wide variety of products. They further note that the majority of transition metal catalysis involves the use of just one metal atom, but there have been exceptions in which catalysts have a two-metal atom core. In this new effort, the researchers used a diatomic Ni-Ni catalyst to carry out stereo-controlled synthesis of cyclopentenes (rings made of five carbon atoms).
The researchers note that in typical Diels–Alder reactions, a diene and an alkene are allowed to react, resulting in a cyclohexene (a ring made of six carbon atoms). They also note that five-member rings are made in nature in a variety of ways, which suggests they might prove useful if they could be easily synthesized. Prior efforts to do so, however, have not panned out. The problem has been dealing with [4 + 1] reactions—there are difficulties involved with generating them using stable molecules. Also, reactivity with them has proven to be a challenge.
Zhou and Uyeda took a different approach, using a two-metal catalyst instead. They found that in their approach, the two metal cores shared the task of controlling how the reaction occurred and in forming the carbene. This made possible the use of dichloroalkenes, which were more stable than diazo compounds. Additionally, just one of the rhodium centers was responsible for the bond formation with the catalyst—the second modulated the reactivity of the first through the bond. The result was a five-sided cyclopentene.
More information: You-Yun Zhou et al. Catalytic reductive [4 + 1]-cycloadditions of vinylidenes and dienes, Science (2019). DOI: 10.1126/science.aau0364
Journal information: Science
© 2019 Science X Network