April 30, 2018 report
Using an SN1 reaction to make quaternary stereocenters
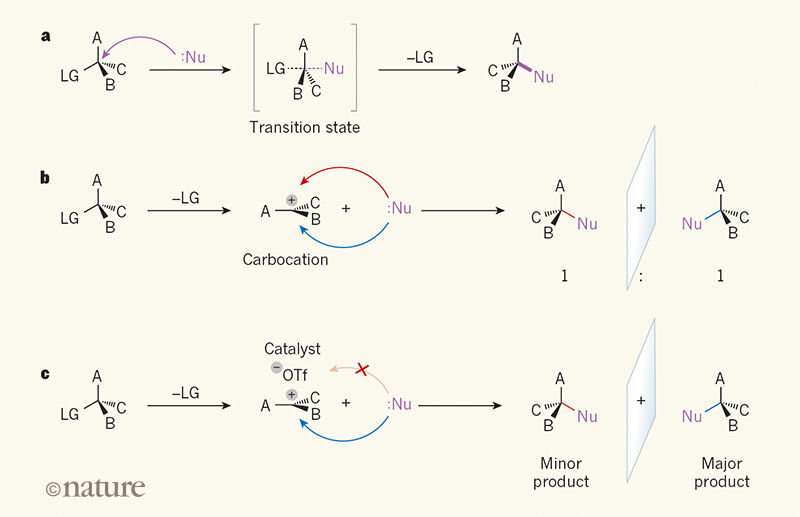
A team of researchers at Harvard University in the U.S. has developed a way to us an SN1 reaction to make quaternary carbon stereocenters. In their paper published in the journal Nature, the team describes overcoming the tendency of SN1 reactions to destroy stereochemistry to make the carbon-centered material. Tobias Morack and Ryan Gilmour from the University of Münster in Germany, offer a News & Views piece on the study in the same journal issue.
As the researchers note, up until now, there has been no good method to make quaternary stereocenters. Current methods, they note, tend to be based on pro-chiral substrates, which are themselves a challenge to make. In this new effort, the team at Harvard came up with a new approach—one based on the SN1 reaction.
SN1 reactions are a mainstay of introductory organic chemistry courses, but they notoriously cause havoc with stereochemistry. In spite of that, the researchers believed they could use the flat carbocation intermediate produced in such reactions as a substrate. To that end, they came up with an SN1 reaction that converted a mixture propargyl acetate (with equal amounts of left and right-handed enantiomers-a racemic) to a mixture with a quaternary center. A hydrogen-bond donor was used as a catalyst to remove the acetoxy materials which were replaced with an allyl mix—the result was a single enantiomer.
The end products were interesting, Morack and Gilmour note, because the carbon stereocenters had a wide range of electron orbitals. This means that they have different physical geometries and thus react differently. This opens up the door to using them to produce a wide variety of molecules that could be used in various reactions for synthesizing new chemicals.
The researchers note that they are not done with the work—they plan to try the same approach with compounds that are not as stable. Doing so, they acknowledge, will require the use of a catalyst that is even more reactive. They also plan to look into conducting the reaction with different nucleophiles.
More information: Alison E. Wendlandt et al. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction, Nature (2018). DOI: 10.1038/s41586-018-0042-1
Abstract
The unimolecular nucleophilic substitution (SN1) mechanism features prominently in every introductory organic chemistry course. In principle, stepwise displacement of a leaving group by a nucleophile via a carbocationic intermediate enables the construction of highly congested carbon centres. However, the intrinsic instability and high reactivity of the carbocationic intermediates make it very difficult to control product distributions and stereoselectivity in reactions that proceed via SN1 pathways. Here we report asymmetric catalysis of an SN1-type reaction mechanism that results in the enantioselective construction of quaternary stereocentres from racemic precursors. The transformation relies on the synergistic action of a chiral hydrogen-bond-donor catalyst with a strong Lewis-acid promoter to mediate the formation of tertiary carbocationic intermediates at low temperature and to achieve high levels of control over reaction enantioselectivity and product distribution. This work provides a foundation for the enantioconvergent synthesis of other fully substituted carbon stereocentres.
Journal information: Nature
© 2018 Phys.org





















