Chemicals can change their identity, thanks to the liquids where they reside
Toss a few whole almonds in a jar full of hazelnuts. Shake. The nuts bounce against each other, but they don't react. That's how some people think of reactions happening inside liquids. The solutes (almonds) react with each other in a sea of solvent (hazelnuts). But a new study shows that this is not always the case for real chemical reactions. Under the right conditions, the solvent can change the chemical identity of the solute.
Many chemical reactions, particularly those relevant to keeping people and plants alive, happen in solution. This research shows that in many such reactions, the solvent is not a mere spectator. That means retooling expectations and computational models. Because the same rules could apply in chemistry labs, researchers may need to select their solvents with more care. The solvents could be controlling or changing the chemical identity of the solute.
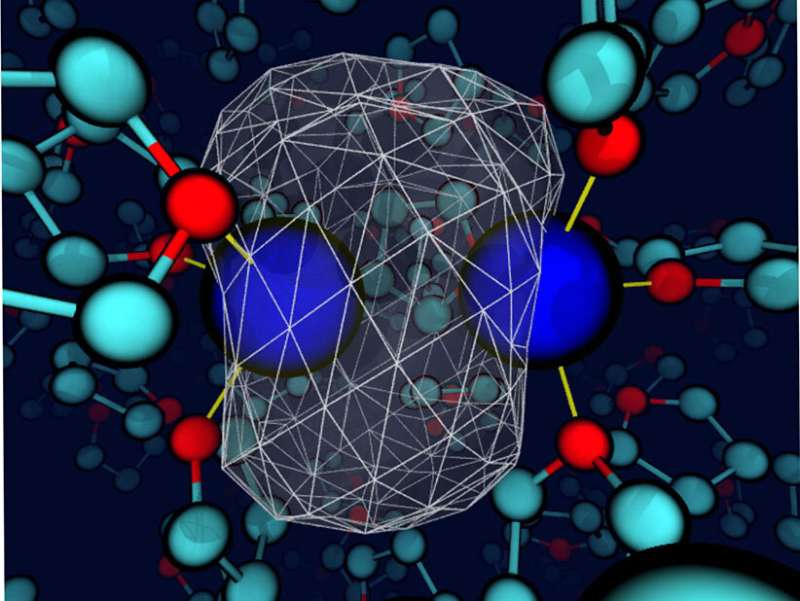
Although solvents are carefully selected in some cases, the liquids are often simply considered a medium to allow the reactants to encounter each other. However, the solvent may play a larger role. Here, researchers found that when the solvent and solute interact (energetically on the same order as a hydrogen bond), the solvent can control the bond dynamics and the chemical identity of simple solutes. The researchers came to this conclusion studying a sodium dimer in the weakly polar solvent tetrahydrofuran. Bonding interactions between the solvent and sodium atoms led to unique coordination states. These states had to cross a free energy barrier, essentially undergoing a chemical reaction, to interconvert. Further, each coordination state had its own dynamics and spectroscopic signatures. Although chemists have long been aware of the influence of solvents in certain cases, this research highlights the value of carefully selecting the solvent to create a specific environment in certain condensed-phase chemical systems.
More information: Devon. R. Widmer et al. Solvents can control solute molecular identity, Nature Chemistry (2018). DOI: 10.1038/s41557-018-0066-z
Journal information: Nature Chemistry
Provided by US Department of Energy