Scientists identify gene cluster in budding yeasts with major implications for renewable energy
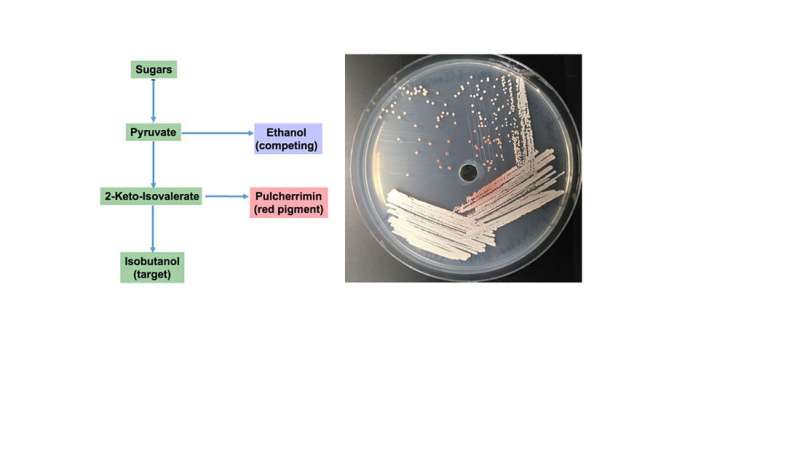
Yeasts are complex organisms that may become the workhorses of biofuel production. To move yeasts into this larger role, scientists need to understand the genetic machinery that leads to the production of complex molecules like the iron-binding molecule pulcherrimin in budding yeasts. Scientists revealed a four-gene cluster associated with pulcherrimin production. Further tests revealed likely functions for each of the genes: two biosynthetic enzymes, a transporter, and a transcription factor involved in both biosynthesis and transport.
Yeasts use the same pathway to make pulcherrimin and an alcohol, isobutanol, of interest for biofuels. Some yeast strains direct a significant amount of carbon into pulcherrimin. Since both pulcherrimin and isobutanol are made from a common pathway, this suggests that the metabolic control of high pulcherrimin producers may be harnessed for increased isobutanol production in yeast. This could help engineer yeast to make larger quantities of isobutanol.
Despite the discovery of an iron-binding pigment known as pulcherrimin 65 years ago, the genes responsible for its biosynthesis remained uncharacterized. Using a comparative genomics approach among 90 genomes from the budding yeast subphylum Saccharomycotina, researchers from the Great Lakes Bioenergy Research Center identified the first yeast secondary metabolite gene cluster and showed that it's responsible for pulcherrimin biosynthesis.
Targeted gene disruptions in Kluyveromyces lactis identified putative functions for each of the four genes: two pulcherriminic acid biosynthesis enzymes, a pulcherrimin transporter, and a transcription factor involved in both biosynthesis and transport. The requirement of a functional putative transporter to utilize extracellular pulcherrimin-complexed iron demonstrates that pulcherriminic acid is a siderophore, an iron-chelating compound secreted by microorganisms. This research also characterized and named two genes that previously lacked assigned functions in the fuel-producing model yeast Saccharomyces cerevisiae. The evolution of this gene cluster in budding yeast suggests an ecological role for pulcherrimin akin to other microbial public goods systems.
Because some yeasts species are particularly adept at funneling carbon into pulcherrimin, studying how high-level pulcherrimin producing strains are altered in their metabolic control may inform strategies for increased biofuel production in model organisms.
More information: David J. Krause et al. Functional and evolutionary characterization of a secondary metabolite gene cluster in budding yeasts, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1806268115
Journal information: Proceedings of the National Academy of Sciences
Provided by US Department of Energy