Mutations in key protein that oversees cellular functions crucial to health and survival
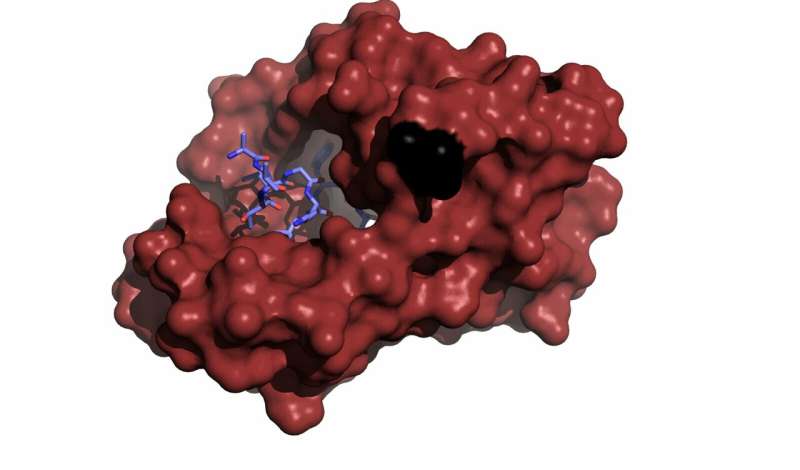
A new research project uses the Canadian Light Source to help researchers understand the protein responsible for regulating heartbeats. Errors in this crucial protein's structure can lead to potentially deadly arrhythmias, and understanding its structure should help researchers develop treatments.
This protein, calmodulin (CaM), regulates the signals that cause the heart to contract and relax in almost all animals with a heartbeat.
"Usually you find some differences between versions of proteins from one species to another," explains Filip Van Petegem, a professor in the University of British Columbia's Department of Biochemistry and Molecular Biology. "For calmodulin that's not the case—it's so incredibly conserved."
It also oversees hundreds of different proteins within the body, adjusting a broad array of cellular functions that are as crucial to our survival and health as a steady heartbeat.
"Because it plays such a crucial role in the heart, interacting with and regulating so many different important proteins, and seeing how calmodulin is identical in all vertebrate species, the general thinking was that you could never have any mutation in your calmodulin genes and survive," says Van Petegem. "You would affect so many different processes in the cell."
But in the last few years, several individuals have been identified who are surviving despite mutations in their CaM genes. While this condition was expected to lead to dire consequences such as epilepsy and major genetic disorders, the most serious symptom appears to be cardiac arrhythmia, an irregular heartbeat that kills 40,000 Canadians a year.
"A lot of people didn't believe it initially, but this has been confirmed," he observes, adding that the findings have raised entirely new questions about just how CaM goes about its business in the body.
Van Petegem and his colleagues turned to the CLS to pin down what various mutations did to the inner workings of this complex protein.
"It's all about getting 3-D structures, and you need very bright X-rays to see a lot of the details in these structures," he explains. "We were also particularly fortunate to have an excellent collaboration with Drs. Overgaard and Wimmer at the University of Aalborg, Denmark, who complemented our studies with nuclear magnetic resonance spectroscopic data".
The result, published in the Proceedings of the National Academy of Sciences, offered further surprises from CaM, which contains upward of 150 amino acids. A single change to any one of these constituents had a huge impact on the shape of the molecule.
"Normally when calmodulin binds to calcium it changes its shape, which is what sends the signal to the calcium channel to shut down, and controls the heartbeat" says Van Petegem, who witnessed a tremendous distortion when a mutation prevented this binding process. "It wasn't a subtle thing—the way the entire protein folds is very different."
Van Petegem's team looked at four different mutant variations of the heartbeat-regulating CaM protein, finding different structures for each.
"Although the different mutations all lead to cardiac arrhythmia, the way they do this is mutation-specific," says Van Petegem. Two of the four mutants expressed large structural conformations, with different consequences shown in the other two.
The mutated structures looked nothing like the CaM found in a healthy individual, yet the protein seemed to still be able to fulfill most of its functions in the body, even if its control over heartbeat is compromised.
This puzzling insight simply adds to the complexities of CaM, which Van Petegem has been pursuing since starting his UBC laboratory in 2007. For now, he continues to weigh the implications of the imagery provided by the synchrotron.
"We would never have predicted beforehand what we saw," he concludes.
More information: Kaiqian Wang et al. Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1808733115
Journal information: Proceedings of the National Academy of Sciences
Provided by Canadian Light Source
















