From a plant sugar to toxic hydrogen sulfide
In a doctoral research project conducted at the Department of Biology, researchers have described the degradation of the dietary sugar sulfoquinovose by anaerobic bacteria to toxic hydrogen sulfide for the first time—increased production of hydrogen sulfide in the human intestinal system has been associated with inflammatory bowel disease and colon cancer.
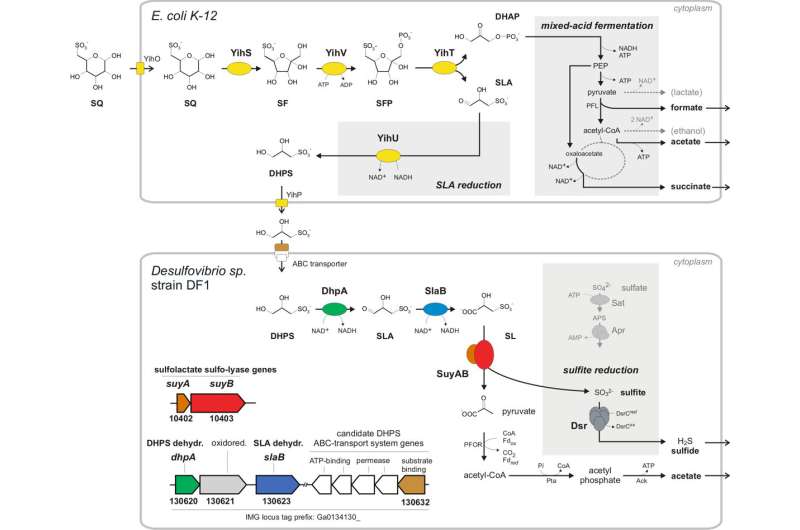
Sulfoquinovose, a sugar found in plants, contains sulfur. A constituent of green-vegetable diets, it is also found in the human intestinal system, an environment without oxygen. Doctoral researcher Anna Burrichter wanted to determine what happens when anaerobic bacteria degrade sulfoquinovose in the absence of oxygen. She discovered a new type of metabolism that transforms sulfoquinovose into hydrogen sulfide (H2S).
These results have been obtained from a laboratory model system. In future studies, researchers will have to examine whether sulfoquinovose in the intestine is actually metabolized to hydrogen sulfide, a toxic compound for humans. The study was conducted by the research team of Dr. David Schleheck, and the results have been published in the current issue of the journal Frontiers in Microbiology.
Anna Burrichter has discovered an entirely novel bacterial degradation pathway that involves three individual discoveries: a new link in the biological sulfur cycle, a new type of fermentation in Escherichia coli, and a previously unknown energy metabolism in sufite-respiring bacteria of the Desulfovibrio species.
"Without oxygen, the degradation pathways are completely different. In the context of sulfoquinovose, we discovered a novel type of fermentation in Escherichia coli," says Anna Burrichter. Along with dihydroxypropane sulfonate, the sulfur-containing degradation product that is formed in this first degradation step, the researchers found a second bacterium, Desulfovibrio, which can utilize this intermediate for anaerobic respiration mode, the so-called sulfite reduction. This type of respiration with organically-bound sulfur as electron acceptor instead of oxygen is described in detail for the first time in Anna Burrichter's thesis. "We thought that hydrogen sulfide may be the end product, but it had never been proven before, and no one knew which bacteria and enzymes may catalyse these reactions," the biologist says.
The next step is to transfer the results of the laboratory model to the human intestine. "We want to investigate if these degradation pathways can also be found in the intestine and how much they contribute to the overall production of hydrogen sulfide, depending on the diet," says David Schleheck.
Previously, it was assumed that organosulfonate substrates, such as taurine, are transformed into hydrogen sulfide mainly from meat-rich and high-fat diets. The new findings now suggest that organosulfonates from vegetarian food, that is sulfoquinovose, can be degraded to hydrogen sulfide, as well.
Burrichter's doctoral thesis is a promising basis for further research in this area. "Now we know the individual steps of the degradation pathway and the enzymes and genes involved, and therefore, we know what we have to search for," says Burrichter. The production of hydrogen sulfide in general can contribute to inflammatory bowel disease and colon cancer. However, it is also assumed that hydrogen sulfide, at least at low concentrations in the intestine, could as well have beneficial effects for health.
David Schleheck says, "To better understand the human microbiome and the effects of hydrogen sulfide, it is essential to know all the pathways that can lead to hydrogen sulfide production. Only then one might be able to better manage the production of hydrogen sulfide in the intestine."
More information: Anna Burrichter et al. Anaerobic Degradation of the Plant Sugar Sulfoquinovose Concomitant With H2S Production: Escherichia coli K-12 and Desulfovibrio sp. Strain DF1 as Co-culture Model, Frontiers in Microbiology (2018). DOI: 10.3389/fmicb.2018.02792
Provided by University of Konstanz