Chemical engineers develop new theory to build improved nanomaterials
Thanks in part to their distinct electronic, optical and chemical properties, nanomaterials are utilized in an array of diverse applications from chemical production to medicine and light-emitting devices. But when introducing another metal in their structure, also known as "doping," researchers are unsure which position the metal will occupy and how it will affect the overall stability of the nanocluster, thereby increasing experimental time and costs.
However, researchers from the University of Pittsburgh's Swanson School of Engineering have developed a new theory to better predict how nanoclusters will behave when a given metal is introduced to their structure. The study, "Thermodynamic Stability of Ligand-Protected Metal Nanoclusters" (DOI: 10.1021/acs.jpclett.8b02679) was featured on the cover of the ACS Journal of Physical Chemistry Letters. Co-authors are Giannis Mpourmpakis, the Bicentennial Alumni Faculty Fellow and Assistant Professor of Chemical and Petroleum Engineering at the Swanson School, and Ph.D. candidate and NSF Graduate Fellow Michael Taylor. Their findings connect with previous research focused on designing nanoparticles for catalytic applications.
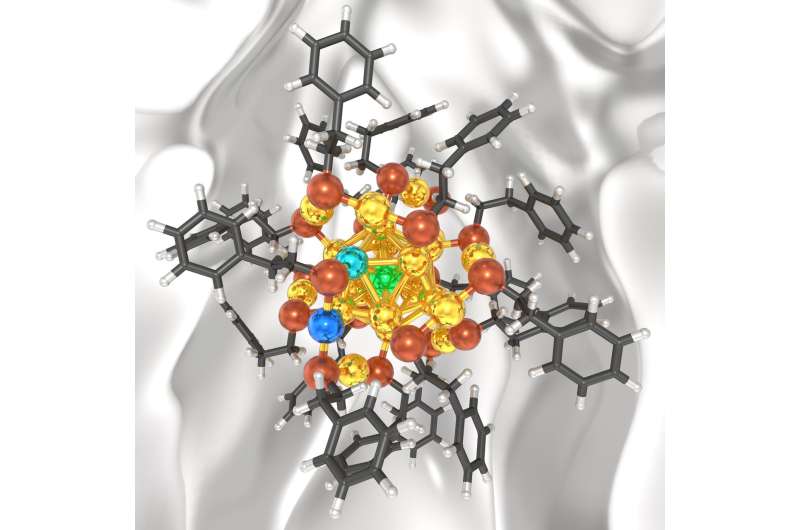
"Engineering the size, shape and composition of nanoclusters is a way to control their inherent properties" Dr. Mpourmpakis said. "In particular, Ligand-protected Au (gold) nanoclusters are a class of nanomaterials where the precise control of their size has been achieved. Our research aimed to better predict how their bimetallic counterparts are being formed, which would allow us to more easily predict their structure without excess trial and error experimentation in the lab."
The research, completed in Dr. Mpourmpakis' Computer-Aided Nano and Energy Lab (C.A.N.E.LA.), enabled them to computationally predict the exact dopant locations and concentrations in ligand-protected Au nanoclusters. They also discovered that their recently developed theory, which explained the exact sizes of experimentally synthesized Au nanoclusters, was also applicable to bimetallic nanoclusters, which have even greater versatility.
"This computational theory can now be used to accelerate nanomaterials discovery and better guide experimental efforts," Dr. Mpourmpakis said. "What's more, by testing this theory on bimetallic nanoclusters we have the potential to develop materials that exhibit tailored properties. This could have a tremendous impact on nanotechnology."
More information: Michael G. Taylor et al, Rethinking Heterometal Doping in Ligand-Protected Metal Nanoclusters, The Journal of Physical Chemistry Letters (2018). DOI: 10.1021/acs.jpclett.8b02679
Journal information: Journal of Physical Chemistry Letters
Provided by University of Pittsburgh