Scouting out bacterial defences to find new ways to counter-attack antibiotic resistance
Research led by the University of Bristol has begun to unpick an important mechanism of antibiotic resistance and suggest approaches to block this resistance.
Antibiotic resistance is the ability of bacteria to defend against antibiotic attack, and the spread of these resistance mechanisms amongst bacteria is a global public health concern. A form of resistance caused by a family of bacterial proteins, the Verona Imipenemase (VIM) beta-lactamases, is of acute clinical concern because it can inactivate antibiotics (penicillins and related agents) that comprise over half of the global antibacterial market.
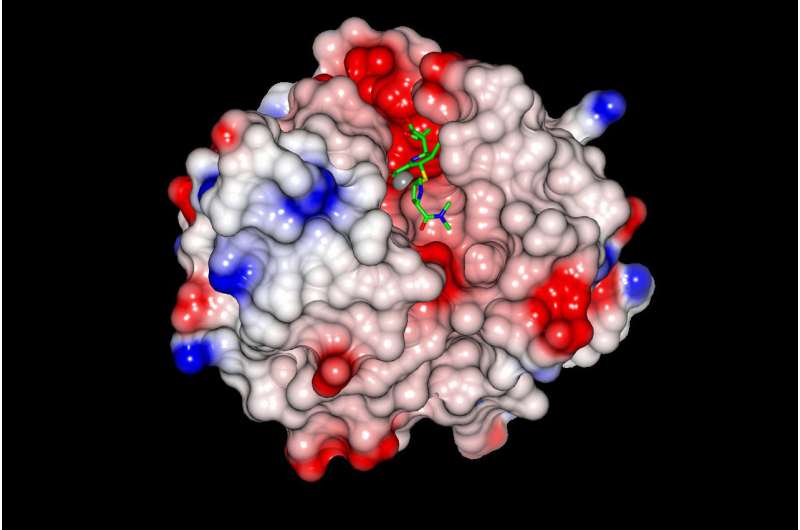
A team of researchers led by the University of Bristol have uncovered near-atomic level structural detail of VIM proteins. The research is published today [Thursday 15 November] in The FEBS Journal.
Dr Jim Spencer, Reader in Microbiology in the School of Cellular and Molecular Medicine at the University of Bristol, who led the research, said: "Our work explains how the products of one family of resistance genes recognise penicillin-type antibiotics and suggests routes to blocking this resistance in future treatments."
The VIM proteins protect bacteria from the most clinically important group of antibiotics, beta-lactams, by binding and subsequently inactivating them, preventing their attack on target bacteria. To thwart VIM protein mediated resistance, the team focused their research on identifying exactly how the VIM proteins bind to the antibiotics.
Dr Spencer added: "We sought to understand how VIM recognises its target antibiotics."
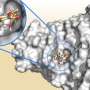
To understand antibiotic binding the researchers needed to examine VIM proteins at an atomic-level. A protein's atomic arrangement can be determined by firing X-rays at the protein of interest and observing the way in which the X-rays are scattered. The necessary high intensity X-rays are produced in particle accelerators called synchrotrons. The research team used the UK's national synchrotron science facility, Diamond Light Source, at the Harwell Science and Innovation Campus in Oxfordshire.
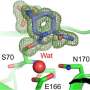
Surprising variation had previously been identified in two specific regions of the VIM proteins, making it hard to explain how different VIM family members could all bind antibiotics. The team were particularly interested in explaining this phenomenon. By collecting near-atomic level crystallographic data on one member of the VIM protein family, the researchers were able to identify a key component of the antibiotic binding mechanism. Structural comparisons with other VIM protein family members confirmed this to be a common feature within the VIM family.
Dr Spencer explained: "The VIM beta-lactamases are a family of enzymes that vary from one another in the region responsible for antibiotic binding, our work explains how antibiotics can bind to different types of VIM beta-lactamase despite these variations.
"Knowledge of the mechanisms by VIM beta-lactamases bind antibiotics will enable researchers to replicate these interactions in molecules designed to block their activity, and so reverse antibiotic resistance."
The research team are now using the information they've gathered to design molecules that bind to VIM proteins.
More information: Crystal Structures of VIM-1 Complexes Explain Active Site Heterogeneity in VIM-Class Metallo-β-Lactamases, The FEBS Journal
Provided by University of Bristol











.jpg)