Sea snail shells dissolve in increasingly acidified oceans, study shows
Shelled marine creatures living in increasingly acidified oceans face a fight for survival as the impacts of climate change spread, a new study suggests.
Researchers from the University of Tsukuba, Japan, and the University of Plymouth, UK, assessed the impact of rising carbon dioxide levels on the large predatory "triton shell" gastropod (Charonia lampas).
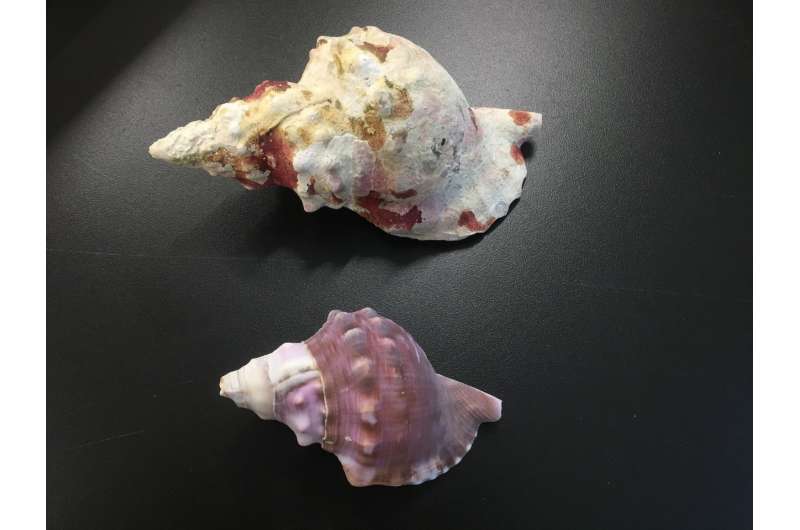
They found those living in regions with predicted future levels of CO2 were on average around a third smaller than counterparts living in conditions seen throughout the world's oceans today.
However there was also a noticeable negative impact on the thickness, density, and structure of their shells, causing visible deterioration to the shell surface.
Writing in Frontiers in Marine Science, scientists say the effects are down to the increased stresses placed on the species in waters where the pH is lower, which reduce their ability to control the calcification process.
And they have warned other shellfish are likely to be impacted in the same way, threatening their survival and that of other species that rely on them for food.
Dr. Ben Harvey, Assistant Professor in the University of Tsukuba's Shimoda Marine Research Center, said: "Ocean acidification is a clear threat to marine life, acting as a stressor for many marine animals. Here we found that the ability of the triton shells to produce and maintain their shells was hindered by ocean acidification, with the corrosive seawater making them smoother, thinner, and less dense. The extensive dissolution of their shells has profound consequences for calcified animals into the future as it is not something they can biologically control, suggesting that some calcified species might be unable to adapt to the acidified seawater if carbon dioxide emissions continue to rise unchecked."
The research was conducted at a marine volcanic seep off the coast of Shikine-jima in Japan where carbon dioxide bubbling up through the seabed lowers seawater pH from present-day levels to future predicted levels.
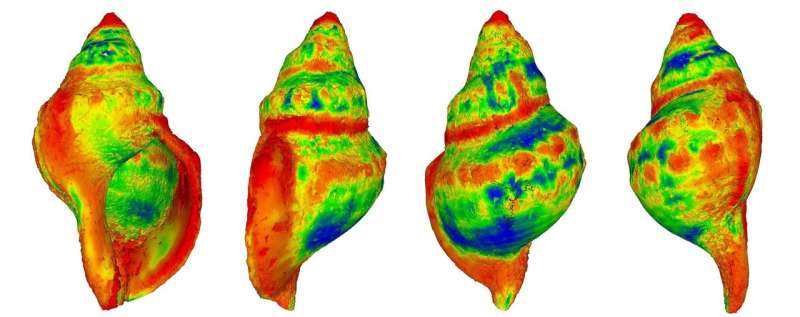
Using computed tomography (CT) scanning, the scientists measured the thickness, density and structure of the shells, with shell thickness halved in areas with raised CO2 while average shell length was reduced from 178mm in sites with present day levels to 112mm.
In some cases, these negative effects left body tissue exposed and the shell casing dissolved, with the corrosive effects of acidi?cation far more pronounced around the oldest parts of the shell.
Jason Hall-Spencer, Professor of Marine Biology at the University of Plymouth, added: "Our study clearly shows that increasing carbon dioxide levels cause seawater to become corrosive to shellfish. As these calcified animals are a fundamental component of coastal marine communities, ocean acidi?cation is expected to impact shellfish fisheries."
More information: Ben P. Harvey et al, Dissolution: The Achilles' Heel of the Triton Shell in an Acidifying Ocean, Frontiers in Marine Science (2018). DOI: 10.3389/fmars.2018.00371
Provided by University of Plymouth